NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
The antiandrogens are medications that act by binding to intracellular androgen receptors, preventing the effects of endogenous androgens on target tissues such as testes, hair follicles, hypothalamus, pituitary, ovaries and prostate gland. The antiandrogens are used for a variety of hyperandrogenic states such as acne, hirsutism, and paraphilias, but their major use is in therapy of prostate cancer. Chemically, antiandrogens are classified into steroidal (such as cyproterone acetate) and nonsteroidal (including flutamide, nilutamide and bicalutamide). Cyproterone, despite evaluation in many clinical trials, has yet to receive FDA approval for use the United States, largely because of its potential for hepatotoxicity. In contrast, flutamide, nilutamide and bicalutamide are now approved and in general clinical use for therapy of prostate cancer. The largest and longest clinical experience has been with flutamide which has been shown to produce clinical remission in patients with prostate cancer, and is also used in hyperandrogenic states including acne and hirsutism. Nilutamide has been extensively studied and is indicated for use in combination with surgical castration for the treatment of metastatic prostate cancer and is recommended to be started at the time of orchiectomy. Unlike flutamide, nilutamide has not been evaluated in detail as therapy of hirsutism or acne. Bicalutamide, the most recent antiandrogen to be approved for use in the United States, is also used in therapy of prostate cancer, but has not been approved for use in nonmalignant hyperandrogenic states.
All three nonsteroidal antiandrogens have been linked to instances of liver injury. Minor asymptomatic elevations in serum aminotransferase levels occur in 10% to 62% of patients on long term antiandrogen therapy, and prominent elevations (3 to 5 times the ULN) in 1% to 10%, the rates being somewhat higher for flutamide than nilutamide or bicalutamide. Instances of acute hepatitis with jaundice and even acute liver failure have been reported with all three antiandrogens. While most reports have related to flutamide, this may be due to its more extensive use. The rate of clinically apparent acute liver injury during prolonged antiandrogen therapy is reported to be as high as 9%, but is probably in the range of 0.1% to 1%. Screening for serum aminotransferase levels during the first few months of therapy is often recommended, but the effectiveness of this approach in preventing serious liver injury and death has not been shown.
Abiraterone is a more recently developed antiandrogen used to treat prostate cancer. Abiraterone is a steroid inhibitor of CYP17, an enzyme in the pathway of androgen production which has been shown to be effective in prolonging relapse free and overall survival in men with metastatic, castration-resistant prostate cancer. Abiraterone has been linked to a moderate rate of serum aminotransferasae elevations during therapy, but the abnormalities are usually mild and transient, and instances of clinically apparent liver injury have not been linked to use of this agent, although its general use has been limited.
Each of the antiandrogens is discussed separately with individual clinical cases and references. The following are links to each drug record.
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Abiraterone | 154229-19-3 | C24-H31-N-O |
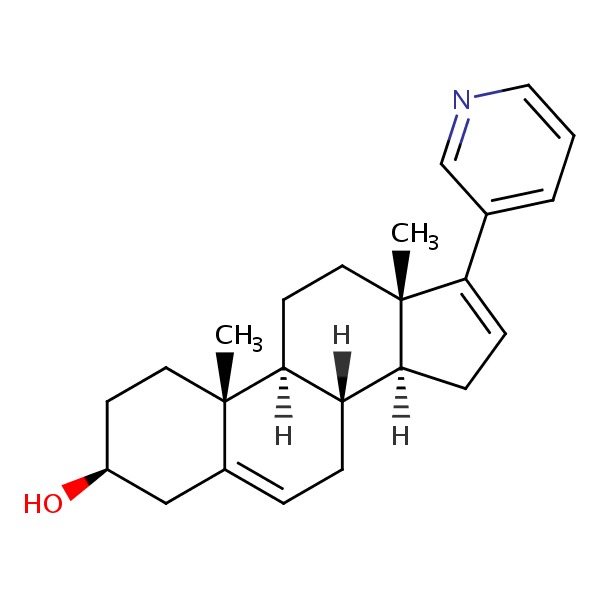 |
| Bicalutamide | 90357-06-5 | C18-H14-F4-N2-O4-S |
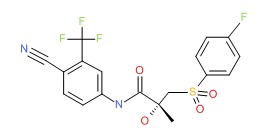 |
| Flutamide | 13311-84-7 | C11-H11-F3-N2-O3 |
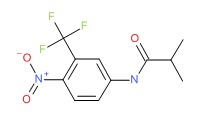 |
| Nilutamide | 63612-50-0 | C12-H10-F3-N3-O4 |
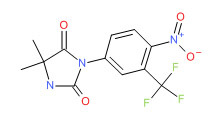 |
ANNOTATED BIBLIOGRAPHY
References updated: 10 June 2014
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, p. 699.(Expert review of hepatotoxicity published in 1999, mentions that flutamide has led to instances of severe hepatic necrosis and fulminant hepatic failure).
- DeLeve LD. Flutamide. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier. 2013. pp. 559.(Textbook on drug induced liver injury mentions that flutamide has been implicated in at least 46 cases of severe liver injury with 20 fatalities).
- Moy B, Lee RJ, Smith M. Hormone therapy in prostate cancer. Natural products in cancer chemotherapy: hormones and related agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1763-9.(Textbook of pharmacology and therapeutics; discusses the role of androgen receptor blockers in prostate cancer including bicalutamide, flutamide and nilutamide).
- Meijers WH, Willemse PH, Sleijfer DT, Mulder NH, Grond J. Hepatocellular damage by cyproterone acetate. Eur J Cancer Clin Oncol 1986; 22: 1121-2. [PubMed: 2946585](In a study of 20 women with breast cancer, cyproterone was given in dose of 200-400 mg/day for 6 to 52 weeks; 5 developed ALT elevations, and two had acute hepatitis after 11 and 21 weeks, with ALT >10 times ULN and liver biopsies showing confluent necrosis).
- Lévesque H, Trivalle C, Manchon ND, Vinel JP, Moore N, Hémet J, Courtois H, et al. Fulminant hepatitis due to cyproterone acetate. Lancet 1989; 1: 215-6. [PubMed: 2563116](78 year old man with prostate cancer developed jaundice 5 months after starting cyproterone [bilirubin 10.4 mg/dL, ALT 1015 U/L, Alk P 153 U/L], progressing to hepatic failure and death within 2 weeks of initial presentation).
- Parys BT, Hamid S, Thomson RG. Severe hepatocellular dysfunction following cyproterone acetate therapy. Br J Urol 1991; 67: 312-3. [PubMed: 1827039](3 case reports of jaundice developing in men [ages 65 to 83 years] with prostate cancer given cyproterone for 9-12 months [bilirubin 19, 10.4, and 16.4 mg/dL; AST 254, 377 and 155 U/L; Alk P 218, 252 and 79 U/L], resolving within 12 weeks of stopping in 1 but progression to liver failure and death in the other 2 patients).
- McLeod DG. Tolerability of nonsteroidal antiandrogens in the treatment of advanced prostate cancer. Oncologist 1997; 2: 18-27. [PubMed: 10388026](Overview of the safety and side effects of the nonsteroidal antiandrogens; ALT elevations occur in 5-10% receiving drug vs 1-3% with placebo; clinically apparent hepatitis can occur and can be fatal; estimated rates being 3/10,000 treated).
- Friedman G, Lamoureux E, Sherker AH. Fatal fulminant hepatic failure due to cyproterone acetate. Dig Dis Sci 1999; 44: 1362-3. [PubMed: 10489919](81 and 66 year old men with prostate cancer developed jaundice 7 and 8 months after starting cyproterone [bilirubin 30 and 12.2 mg/dL, ALT 395 and 702 U/L, Alk P 376 and 144 U/L], with progression and fatal outcome in both. Review of 7 published cases; onset 2-15 [mean=8] months, bilirubin 9-30 times ULN, all hepatocellular).
- Pu YS, Liu CM, Kao JH, Chen J, Lai MK. Antiandrogen hepatotoxicity in patients with chronic viral hepatitis. Eur Urol 1999; 36: 293-7. [PubMed: 10473987](Retrospective study of 121 men receiving antiandrogen therapy with flutamide or cyproterone found abnormal ALT levels in most or all who were HBsAg or anti-HCV positive; but many were not tested and frequency of ALT monitoring was unclear. Authors propose that antiandrogen hepatotoxicity is more frequent among men with preexisting hepatitis B or C, but no attempt was made to separate hepatotoxicity from exacerbation of underlying disease).
- Lin AD, Chen KK, Lin AT, Chang YH, Wu HH, Kuo JY, Huang WJ, et al. Antiandrogen-associated hepatotoxicity in the management of advanced prostate cancer. J Chin Med Assoc 2003; 66: 735-40. [PubMed: 15015823](Retrospective review of 229 patients with prostate cancer treated with flutamide or cyproterone combined with a luteinizing hormone-releasing hormone agonist, orchiectomy or radiotherapy with regular monitoring of ALT: ALT >2 times ULN occurred in 15% of flutamide and 9.5% CPA recipients; ALT >6 times ULN in 5% vs 4%; no deaths, a few had jaundice).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the United States between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but no case was attributed to an antiandrogen, perhaps because patients with prostate cancer would not be eligible for liver transplantation).
- Thole Z, Manso G, Salgueiro E, Revuelta P, Hidalgo A. Hepatotoxicity induced by antiandrogens: a review of the literature. Urol Int 2004; 73: 289-95. Review. [PubMed: 15604569](Systematic review of the literature from the Spanish pharmacovigilance group; 21 reports of hepatotoxicity from cyproterone, 46 flutamide, 4 nilumidine, only 1 bicalutamide; 6 cases of hepatocellular carcinoma linked to cyproterone therapy).
- Manso G, Thole Z, Salgueiro E, Revuelta P, Hidalgo A. Spontaneous reporting of hepatotoxicity associated with antiandrogens: data from the Spanish pharmacovigilance system. Pharmacoepidemiol Drug Saf 2006; 15: 253-9. [PubMed: 16294367](Analysis of spontaneous reporting to Spanish Pharmacovigilance system found 88 cases of flutamide, 11 bicalutamide and 15 cyproterone hepatotoxicity, latency 3-6 months; 2 fatalities, both from flutamide).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis 2006; 38: 33-8. (Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports; flutamide ranked 11th with a total of 59 cases) [PubMed: 16054882].
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to an antiandrogen).
- Merwat SN, Kabbani W, Adler DG. Fulminant hepatic failure due to nilutamide hepatotoxicity. Dig Dis Sci 2009; 54: 910-3. [PubMed: 18688719](76 year old man with prostate cancer developed abdominal pain followed by jaundice 6 weeks after starting nilutamide [bilirubin 25.8 mg/dL, ALT 1793 U/L, Alk P 231 U/L, INR 1.7], followed by progressive hepatic and multiorgan failure and death).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 25 from antituberculosis drugs, 9 from sulfonamides, 12 from nitrofurantoin, 6 from antifungal agents, 11 from antiepileptic drugs, and 12 from herbals, but none were attributed to antiandrogen therapies).
- Brahm J, Brahm M, Segovia R, Latorre R, Zapata R, Poniachik J, Buckel E, Contreras L. Acute and fulminant hepatitis induced by flutamide: case series report and review of the literature. Ann Hepatol 2011; 10: 93-8. [PubMed: 21301018](Analysis of 10 patients with flutamide hepatotoxicity seen at a single institution in Chile included 3 men [ages 67-80 with prostate cancer] and 7 women [ages 20-44 with hirsutism], onset after 40 to 180 days, 9 with jaundice [bilirubin 2.4 to 44 mg/dL, ALT 159 to 3360 U/L], 5 progressed to acute liver failure requiring liver transplantation [all women]).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. (Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, including 18 due to cyproterone and 12 to flutamide, 11 from Chile [10 in Brahm 2011] and 1 from Colombia). [PubMed: 24552865]
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, Miller K, et al.; COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152-60. [PubMed: 25601341](Among 1088 patients with metastatic, castration-resistant prostate cancer treated with abiraterone and prednisone vs prednisone alone, side effects included mild ALT elevations [13% vs 5%] that were rarely greater than 5 times ULN [0.7% vs 0%] and there were no instances of clinically apparent liver injury or liver related deaths).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Nilutamide: an antiandrogen for the treatment of prostate cancer.[Ann Pharmacother. 1997]Review Nilutamide: an antiandrogen for the treatment of prostate cancer.Dole EJ, Holdsworth MT. Ann Pharmacother. 1997 Jan; 31(1):65-75.
- Review Antiandrogen treatments in locally advanced prostate cancer: are they all the same?[J Cancer Res Clin Oncol. 2006]Review Antiandrogen treatments in locally advanced prostate cancer: are they all the same?Gillatt D. J Cancer Res Clin Oncol. 2006 Aug; 132 Suppl 1:S17-26.
- Tolerability of Nonsteroidal Antiandrogens in the Treatment of Advanced Prostate Cancer.[Oncologist. 1997]Tolerability of Nonsteroidal Antiandrogens in the Treatment of Advanced Prostate Cancer.McLeod DG. Oncologist. 1997; 2(1):18-27.
- The preclinical development of bicalutamide: pharmacodynamics and mechanism of action.[Urology. 1996]The preclinical development of bicalutamide: pharmacodynamics and mechanism of action.Furr BJ, Tucker H. Urology. 1996 Jan; 47(1A Suppl):13-25; discussion 29-32.
- Review Antiandrogens: a summary review of pharmacodynamic properties and tolerability in prostate cancer therapy.[Arch Ital Urol Androl. 1999]Review Antiandrogens: a summary review of pharmacodynamic properties and tolerability in prostate cancer therapy.Migliari R, Muscas G, Murru M, Verdacchi T, De Benedetto G, De Angelis M. Arch Ital Urol Androl. 1999 Dec; 71(5):293-302.
- Antiandrogens - LiverToxAntiandrogens - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...