NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Molnupiravir is a ribonucleoside analogue and antiviral agent that is used in the therapy the severe acute respiratory syndrome (SARS) coronavirus 2 (CoV-2) infection, the cause of the novel coronavirus disease, 2019 (COVID-19). Molnupiravir therapy is given orally for 5 days early in the course of SARS-CoV-2 infection and has not been linked to serum aminotransferase elevations or to clinically apparent liver injury.
Background
Molnupiravir (mole” nu peer’ a veer) is an orally absorbed, prodrug of the ribonucleoside analogue N-hydroxycytidine (NHC), which has activity in vitro against several coronaviruses including SARS-CoV-1 and -2. Once taken up in the replicating viral genome, NHC can base pair with either cytidine (the correct) or adenine (the incorrect ribonucleotide), thus causing lethal mutagenesis of the replicating virus. The efficacy of this somewhat unique mechanism of inhibition of viral replication depends upon the lack of proofreading by the viral RNA dependent RNA polymerase, and its safety depends upon effective proofreading by host DNA polymerases. Molnupiravir was found to be mutagenic in the Ames mutagenesis assay and in some but not all mammalian assays for host genetic mutations. In preregistration early phase trials, molnupiravir was found to be safe in doses of 400 to 1600 mg twice daily with adverse event rates no greater than with placebo and no apparent dose related toxicity. Trials of molnupiravir started within 5 days of onset of symptoms demonstrated a 30% to 50% reduction in subsequent clinical worsening of COVID-19, such that hospitalization was not required nor did the infection result in death. Based upon these results and the ongoing COVID-19 pandemic, molnupiravir was granted Emergency Use Authorization (EUA) in December 2021 as therapy of non-hospitalized adults with documented COVID-19 infection who are at high risk of complications. Molnupiravir is available under the EUA as 200 mg capsules, the recommended dose being four 200 mg capsules (800 mg) twice daily for 5 days. Longer term therapy is not recommended, nor is therapy recommended for hospitalized patients or patients who have had symptoms or signs for more than 5 days. Currently, molnupiravir is being actively evaluated for efficacy and safety in treating patients not at high risk for complications, for children, and for patients with known exposure to COVID-19 (post-exposure prophylaxis). Molnupiravir appears to be generally well tolerated; mild adverse events may include headache, dizziness, gastrointestinal upset, nausea and diarrhea. Molnupiravir is potentially teratogenic and is contraindicated in pregnancy. A potential side effect is impairment of bone and cartilage growth and it is not recommended for use in children. The total clinical experience with molnupiravir has been limited and its safety not fully defined.
Hepatotoxicity
In preregistration clinical trials, serum aminotransferase elevations were uncommon and mild, and were no more frequent with molnupiravir than with placebo. Furthermore, among more than 900 patients treated with molnupiravir (800 mg twice daily) for 5 days in prelicensure studies, there were no reported episodes of clinically apparent liver injury. Confounding the issue is that serum aminotransferase elevations are common during symptomatic SARS-CoV-2 infection, present in up to 70% of patients and are more frequent in patients with severe disease and in those with the known risk factors for COVID-19 severity such as male sex, older age, higher body mass index and diabetes. Thus, molnupiravir has not been shown to cause liver injury, but the total clinical experience with its use is limited.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The lack of adverse events and hepatic injury from molnupiravir may be due to its lack of major hepatic metabolism and the relatively short duration of therapy. Molnupiravir is a prodrug that is absorbed orally and rapidly metabolized to NHC, which has little hepatic metabolism. Whether long term molnupiravir is also without serious adverse events remains to be seen. Studies in animal models have suggested that longer term therapy may result in thrombocytopenia and bone structural changes. Furthermore, the findings in mutagenesis studies suggest that molnupiravir may cause host DNA damage.
Drug Class: Antiviral Agents
Other Drugs in the Subclass: Paxlovid
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Molnupiravir – Generic
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
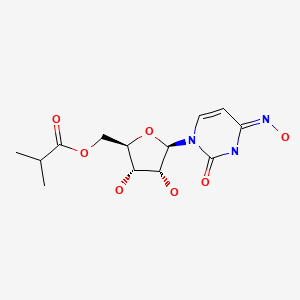
| Molnupiravir | 2492423-29-5 | C13-H19-N3-O7 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 31 January 2022
Abbreviations used: COVID-19, coronavirus disease, 2019; ICU, intensive care unit; IFN, interferon; IL, interleukin; MERS, Middle East respiratory syndrome; NHC, N-hydroxycytidine; SARS-CoV-2, severe acute respiratory syndrome-coronavirus 2.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [PMC free article: PMC7159299] [PubMed: 31986264](Among 41 adults with COVID-19 pneumonia hospitalized in Wuhan China in December 2019-January 2020, 37% had serum AST elevations [62% of those in the ICU and 25% of those not] with concurrent elevations in proinflammatory cytokines [IL1B, IL6, IL2, IFN gamma], and 15% died).
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [PMC free article: PMC7092819] [PubMed: 32109013](Among1099 patients hospitalized with COVID-19 at 552 hospitals in China through January 2020, the median age was 47 years, 42% were women, 2.4% were admitted to an ICU, 1.4% died and ALT elevations arose in 4.1%).
- Qiu H, Wander P, Bernstein D, Satapathy SK. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Liver Int. 2020;40:1590–1593. [PubMed: 32369658](56 year old woman with decompensated alcoholic cirrhosis developed worsening jaundice and liver function when admitted with SARS-CoV-2 infection [bilirubin rising from 9.4 to ~17.8 mg/dL, ALT from 94 to ~275 U/L, AST from 184 to ~880 U/L, Alk P from 128 to ~195 U/L, INR from 1.92 to 2.6], improving back towards baseline as the infection resolved).
- Wander P, Epstein M, Bernstein D. COVID-19 presenting as acute hepatitis. Am J Gastroenterol. 2020;115:941–942. [PMC free article: PMC7172489] [PubMed: 32301760](59 year old woman with HIV infection developed fatigue and jaundice [bilirubin 0.6 mg/dL, ALT 697 U/L, Alk P 145 U/L, INR 1.08] and then developed fever and cough with positive tests for SARS-CoV-1, liver tests falling over the next week as she recovered from COVID-19 ).
- Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, et al. Abnormal liver function tests in COVID-19 patients: relevance and potential pathogenesis. Hepatology. 2020;72(5):1864–1872. [PMC free article: PMC7404414] [PubMed: 32702162](Review of the prevalence and potential causes of abnormal liver tests in patients with SARS-CoV-2 infection, ALT elevations being reported in 4-39% of patients with higher rates in patients with more severe disease).
- Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal liver tests in COVID-19: A retrospective observational cohort study of 1827 patients in a major U.S. hospital network. Hepatology. 2020 Oct;72(4):1169–1176. [PMC free article: PMC9258788] [PubMed: 32725890](Among 1877 patients hospitalized with SARS-CoV-2 infection, serum ALT levels were elevated before hospitalization in 19%, at admission in 42% and a peak during hospitalization in 62%, with 21% being greater than 5 times ULN; elevations correlated with disease severity and its risk factors: male sex, older age, higher BMI and diabetes).
- Painter WP, Holman W, Bush JA, Almazedi F, Malik H, Eraut NCJE, Morin MJ, et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother. 2021;65:e02428–20. [PMC free article: PMC8092915] [PubMed: 33649113](Among 64 healthy adults participating in a double-blind dose and safety trial, adverse events were less frequent with molnupiravir [50 to 1600 mg daily] than placebo and there were no clinically significant findings or dose related trends in laboratory values).
- Zhou S, Hill CS, Sarkar S, Tse LV, Woodburn BMD, Schinazi RF, Sheahan TP, et al. β-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but Is also mutagenic to mammalian cells. J Infect Dis. 2021;224:415–419. [PMC free article: PMC8136050] [PubMed: 33961695](In cell culture, N-hydroxycytidine [NHC] had potent [EC50 0.3 µM] activity against SARS-CoV-2 replication and created mutant viral genomes in a dose dependent manner, while ribavirin and favipiravir had minimal effects on replication and only at high concentrations; NHC also resulted in mutations in host genes in a mammalian cell culture system while ribavirin and favipiravir had minimal or no mutagenic effects).
- Gordon CJ, Tchesnokov EP, Schinazi RF, Götte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem. 2021 Jul;297:100770. [PMC free article: PMC8110631] [PubMed: 33989635](Studies with the SARS-CoV-2 RNA dependent RNA polymerase complex suggested that molnupiravir causes mutations in viral genomes by incorporation into the viral RNA template).
- Kabinger F, Stiller C, Schmitzová J, Dienemann C, Kokic G, Hillen HS, Höbartner C, et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28:740–746. [PMC free article: PMC8437801] [PubMed: 34381216](In vitro assays demonstrated that molnupiravir is converted to N-hydroxycytidine [NHC] which can be used by the SARS-CoV-2 RNA dependent RNA polymerase to create negative strand viral RNA which is then used as a template for positive strand viral RNA, and since NHC can base pair with either cytidine or adenine, it can thus create mutations in subsequent negative strand synthesis).
- Khoo SH, Fitzgerald R, Fletcher T, Ewings S, Jaki T, Lyon R, Downs N, et al. Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study. J Antimicrob Chemother. 2021;76:3286–3295. [PMC free article: PMC8598307] [PubMed: 34450619](Among 18 outpatients with documented SARS-CoV-2 infection started on molnupiravir [300, 400 or 800 mg] or placebo twice daily for 5 days within 5 days of onset, all patients tolerated therapy well with no serious adverse events; 1 of 12 patients on active drug had a mild, transient ALT elevation).
- Fischer WA 2nd, Eron JJ Jr, Holman W, Cohen MS, Fang L, Szewczyk LJ, Sheahan TP, et al. A Phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022;14(628):eabl7430. [PMC free article: PMC10763622] [PubMed: 34941423](Among 202 unvaccinated patients with documented SARS-CoV-2 infection enrolled within 7 days of onset of symptoms who were treated with oral molnupiravir [200, 400 or 800 mg] or placebo twice daily for 5 days, the median time to clearance of nasal swab SARS-CoV-2 RNA was shorter with 800 mg daily [15 days] than placebo [14 days] and adverse event rates were lower with active drug, ALT elevations occurring in 2.8% vs 3.2% and there were no serious adverse events).
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, et al. MOVe-OUT Study Group. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2021 Dec 16; Epub ahead of print. [PMC free article: PMC8693688] [PubMed: 34914868](Among 1433 outpatients at high risk of complications of COVID-19 started on oral molnupiravir or placebo within 5 days of onset of symptoms or signs, rates of hospitalization [6.8% vs 9.7%] and deaths within 29 days [0.1% vs 1.3%] were less with molnupiravir than placebo, while adverse event rates were similar [30%vs 33%]; no mention of ALT elevations or hepatotoxicity).
- Whitley R. Molnupiravir – A step toward orally bioavailable therapies for Covid-19. N Engl J Med. 2021 Dec 16; Epub ahead of print. [PMC free article: PMC8693684] [PubMed: 34914869](Editorial in response to Jayk Bernal [2021] mentions that response rates with molnupiravir were greater in the interim analysis [50%] than the final analysis [30%] of the MOVe-OUT trial, and that starting antiviral therapy early appears to be crucial in achieving a response in COVID-19).
- Couzin-Frankel J. Antiviral pills could change pandemic's course. Science. 2021;374(6569):799–800. [PubMed: 34762459](News report of the promise of antivirals including Paxlovid and molnupiravir as a means of treatment for early SARS-CoV-2 infection; no discussion of adverse events).
- Molnupiravir for treatment of COVID-19. Med Lett Drug Ther. 2022;64:10–11. [PubMed: 35134041](Concise review of the mechanism of action, clinical efficacy, safety and indications of molnupiravir shortly after its Emergency Use Authorization in the US mentions mild adverse events arising during the 5 day treatment including diarrhea, nausea and dizziness and potential risks of embryofetal toxicity and impaired bone and cartilage growth; no mention of ALT levels or liver injury).
- Law MF, Ho R, Law KWT, Cheung CKM. Gastrointestinal and hepatic side effects of potential treatment for COVID-19 and vaccination in patients with chronic liver diseases. World J Hepatol. 2021;13:1850–1874. [PMC free article: PMC8727202] [PubMed: 35069994](Summary of the therapies for COVID-19 therapy and their potential for causing liver injury mentions that ALT elevations have occurred in some patients treated with molnupiravir).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Molnupiravir and Its Antiviral Activity Against COVID-19.[Front Immunol. 2022]Review Molnupiravir and Its Antiviral Activity Against COVID-19.Tian L, Pang Z, Li M, Lou F, An X, Zhu S, Song L, Tong Y, Fan H, Fan J. Front Immunol. 2022; 13:855496. Epub 2022 Apr 4.
- Molnupiravir and risk of post-acute sequelae of covid-19: cohort study.[BMJ. 2023]Molnupiravir and risk of post-acute sequelae of covid-19: cohort study.Xie Y, Choi T, Al-Aly Z. BMJ. 2023 Apr 25; 381:e074572. Epub 2023 Apr 25.
- Population pharmacokinetics of molnupiravir in adults with COVID-19: Lack of clinically important exposure variation across individuals.[CPT Pharmacometrics Syst Pharm...]Population pharmacokinetics of molnupiravir in adults with COVID-19: Lack of clinically important exposure variation across individuals.Bihorel S, Cao Y, Chawla A, Birger R, Maas BM, Gao W, Roepcke S, Sardella S, Humphrey R, Kondragunta S, et al. CPT Pharmacometrics Syst Pharmacol. 2023 Dec; 12(12):1859-1871. Epub 2023 Oct 5.
- Review Paxlovid.[LiverTox: Clinical and Researc...]Review Paxlovid.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Molnupiravir-A Novel Oral Anti-SARS-CoV-2 Agent.[Antibiotics (Basel). 2021]Molnupiravir-A Novel Oral Anti-SARS-CoV-2 Agent.Lee CC, Hsieh CC, Ko WC. Antibiotics (Basel). 2021 Oct 23; 10(11). Epub 2021 Oct 23.
- Molnupiravir - LiverToxMolnupiravir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...