NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Molindone is a conventional antipsychotic agent used in the therapy of schizophrenia. Molindone therapy is commonly associated with minor serum aminotransferase elevations but has rarely been linked to cases of clinically apparent acute liver injury.
Background
Molindone (moe lin' done) is a dihydroindolone antipsychotic medication that is not structurally related to the phenothiazines and which appears to act by blocking dopamine type 2 (D2) receptors. Molindone has other central and peripheral effects including anticholinergic and alpha adrenergic blockade. Molindone was approved for use in the therapy of psychotic disorders in the United States in 1974. Since then, however, molindone has been replaced in large part by the atypical antipsychotics, which have fewer extrapyramidal side effects. Molidone is no longer frequently used, but remains available as tablets of 5, 10, 25 and 50 mg generically and previously under the brand name Moban. Recommended doses of molindone were 50 to 75 mg daily initially, increasing based upon efficacy and tolerance to as high as 225 mg daily. Common side effects included drowsiness, dizziness, headache, blurred vision, dry mouth, and tremor. Less common but potentially severe adverse events include extrapyramidal symptoms, akathisia, tardive dyskinesia, neuroleptic malignant syndrome, leukopenia, agranulocytosis, falls and excess mortality in elderly subjects with dementia.
Hepatotoxicity
Liver test abnormalities have been reported to occur in a small proportion of patients on long term therapy with molindone, but elevations are uncommonly above 3 times the upper limit of normal. The aminotransferase abnormalities are usually mild, asymptomatic and transient, reversing even with continuation of medication. Instances of clinically apparent acute liver injury have been reported due to molindone, but are rare. The onset of injury is within 4 to 8 weeks, and the pattern of serum enzyme elevations is typically hepatocellular. Jaundice is uncommon and most cases are self-limited and mild. Immunoallergic features and autoantibody formation are not typical.
Likelihood score: D (possible, rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which molindone causes serum aminotransferase elevations is not known, but is likely due to production of a toxic intermediate by its metabolism. Molindone is extensively metabolized by the liver via sulfoxidation and oxidation, but has not been implicated in clinically significant drug-drug interactions.
Outcome and Management
The serum aminotransferase elevations that occur on molindone therapy are usually self-limited and do not require dose modification or discontinuation of therapy. No instances of acute liver failure or vanishing bile duct syndrome due to molindone have been reported. Patients with molindone induced liver injury probably do not have cross sensitivity to atypical antipsychotics.
Drug Class: Antipsychotic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Molindone – Generic, Moban®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
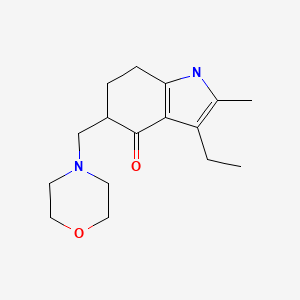
| Molindone | 7416-34-4 | C16-H24-N2-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 21 January 2020
- Zimmerman HJ. Neuroleptic drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 483-91.(Expert review of hepatotoxicity of neuroleptic drugs including molindone published in 1999; mentions that molindone can cause serum aminotransferase elevations and has been linked to rare cases of clinically apparent cases of liver injury).
- Larrey D, Ripault MP. Molindone. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 455.(Review of hepatotoxicity of psychiatric agents mentions mentions a single case of clinically apparent liver injury due to molindone).
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- Kellner R, Rada RT, Egelman A, Macaluso B. Long-term study of molindone hydrochloride in chronic schizophrenics. Curr Ther Res Clin Exp. 1976;20:686–94. [PubMed: 825355](Open label study of molindone for 6 months in 23 patients; 2 had minimal ALT elevations; no details given).
- Bhatia SC, Banta LE, Ehrlich DW. Molindone and hepatotoxicity. Drug Intell Clin Pharm. 1985;19:744–6. [PubMed: 4053979](17 year old male developed fever and fatigue 4 weeks after starting molindone [bilirubin 0.3 mg/dL, ALT 694 U/L, Alk P 100 U/L], resolving within 3 weeks of stopping; rechallenge for 4 days led to ALT elevations [134 U/L]; patient later tolerated thioridazine; authors mention 11 previous instances of hepatotoxicity, all of which were asymptomatic and self-limited).
- Claghorn JL. Review of clinical and laboratory experiences with molindone hydrochloride. J Clin Psychiatry. 1985;46(8 Pt 2):30–3. [PubMed: 3894340](Review of pharmacokinetics, mechanism of action and efficacy of molindone; no mention of hepatic side effects).
- Munyon WH, Salo R, Briones DF. Cytotoxic effects of neuroleptic drugs. Psychopharmacology (Berl). 1987;91:182–8. [PubMed: 2883697](In vitro testing for cytotoxicity suggested that the phenothiazines are the most, haloperidol intermediate and loxapine and molindone the least toxic).
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. [PubMed: 10553730](Systematic review of 81 articles on weight change with antipsychotics, using change after 10 weeks to compare agents: clozapine +5.7, olanzapine +4.2, chlorpromazine +4.2, risperidone +1.7, loxapine +0.6, haloperidol +0.5, ziprasidone +0.3, molindone -0.1, and pimozide -2.7 kg).
- Bagnall A, Fenton M, Kleijnen J, Lewis R. Molindone for schizophrenia and severe mental illness. Cochrane Database Syst Rev. 2007;(1):CD002083. [PubMed: 17253473](Cochrane review of efficacy and safety; no mention of hepatotoxicity or serum ALT elevations).
- Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Ritz L, Ambler D, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420–31. [PubMed: 18794207](Prospective trial of molindone [1st generation] vs olanzapine or risperidone [2nd generation] for schizophrenia in 119 youths found similar rates of efficacy [34-50%], but more weight gain [mean 6.1 kg] and ALT elevations with olanzapine).
- Torrent C, Amann B, Sanchez-Moreno J, Colom F, Feinares M, Comes M, Rosa AR, et al. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand. 2008;118:4–18. [PubMed: 18498432](Review of frequency of weight gain in patients treated for bipolar disorders, most weight gain occurred with clozapine and olanzapine, but some weight gain also with quetiapine, risperidone, lithium, valproate and gabapentin; little or none with molindone, loxapine, carbamazepine and lamotrigine).
- Findling RL, Johnson JL, McClellan J, Frazier JA, Vitiello B, Hamer RM, Lieberman JA, et al. Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum (TEOSS) study. J Am Acad Child Adolesc Psychiatry. 2010;49:583–94. [PMC free article: PMC2882800] [PubMed: 20494268](119 youths with early onset schizophrenia were treated with olanzapine, risperidone or molindone for 8 weeks and 54 were treated in a maintenance study; those on molindone showed the highest numerical increases in ALT [increase of 3-5 U/L], but there were no drug discontinuations for liver disease).
- Stocks JD, Taneja BK, Baroldi P, Findling RL. A phase 2a randomized, parallel-group, dose-ranging study of molindone in children with attention-deficit/hyperactivity disorder and persistent, serious conduct problems. J Child Adolesc Psychopharmacol. 2012;22:102–11. [PubMed: 22372512](Among 78 children with attention deficit syndrome treated with 1 of 4 doses of molindone for 12 weeks, adverse events included somnolence, weight gain, akathisia, sedation and abdominal pain, and there were “no clinically meaningful changes in laboratory” findings).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to antipsychotic drugs or molindone).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to molindone or other antipsychotic drugs).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. PMID: 25754159. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 were attributed to antipsychotic agents, including olanzapine [n=2] and quetiapine [n=3] but not molindone).
- Drugs for psychotic disorders. Med Lett Drugs Ther. 2016;58(1510):160–4. [PubMed: 27960194](Concise review of the mechanism of action, clinical efficacy, toxicity and costs of antipsychotic drugs, mentions that molindone is a first generation agent and is less sedative and less likely to cause extrapyramidal symptoms than haloperidol and the phenothiazines; no mention of ALT elevations or hepatotoxicity).
- Solmi M, Murru A, Pacchiarotti I, Undurraga J, Veronese N, Fornaro M, Stubbs B, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–77. [PMC free article: PMC5499790] [PubMed: 28721057](Safety and tolerability of antipsychotic medications in current use, mentions that liver damage is most common with chlorpromazine, olanzapine, clozapine, quetiapine and risperidone; hepatic injury related to molindone is not discussed).
- Krause M, Zhu Y, Huhn M, Schneider-Thoma J, Bighelli I, Chaimani A, Leucht S. Efficacy, acceptability, and tolerability of antipsychotics in children and adolescents with schizophrenia: A network meta-analysis. Eur Neuropsychopharmacol. 2018;28:659–74. [PubMed: 29802039](Metaanalysis of published controlled trials of antipsychotic medications in children and adolescents mentions that molindone was superior to placebo and to haloperidol in studies of efficacy and was the best agent in terms of weight gain; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Molindone for schizophrenia and severe mental illness.[Cochrane Database Syst Rev. 2000]Review Molindone for schizophrenia and severe mental illness.Bagnall A, Fenton M, Lewis R, Leitner ML, Kleijnen J. Cochrane Database Syst Rev. 2000; (2):CD002083.
- Review Molindone for schizophrenia and severe mental illness.[Cochrane Database Syst Rev. 2007]Review Molindone for schizophrenia and severe mental illness.Bagnall A, Fenton M, Kleijnen J, Lewis R. Cochrane Database Syst Rev. 2007 Jan 24; (1):CD002083. Epub 2007 Jan 24.
- Review Molindone hydrochloride: a review of laboratory and clinical findings.[J Clin Psychopharmacol. 1989]Review Molindone hydrochloride: a review of laboratory and clinical findings.Owen RR Jr, Cole JO. J Clin Psychopharmacol. 1989 Aug; 9(4):268-76.
- Relation of serum molindone levels to serum prolactin levels and antipsychotic response.[J Clin Psychiatry. 1989]Relation of serum molindone levels to serum prolactin levels and antipsychotic response.Pandurangi AK, Narasimhachari N, Blackard WG, Landa BS. J Clin Psychiatry. 1989 Oct; 50(10):379-81.
- Review Loxapine.[LiverTox: Clinical and Researc...]Review Loxapine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Molindone - LiverToxMolindone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...