NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Mitoxantrone is an antineoplastic antibiotic that is used in the treatment of acute leukemia, lymphoma, and prostate and breast cancer, but also for late stage, severe multiple sclerosis. Mitoxantrone therapy is often accompanied by mild to moderate elevations in serum aminotransferase levels, but in typical doses it rarely causes clinically apparent, acute liver injury.
Background
Mitoxantrone (mye tox’ an trone) is an antineoplastic antibiotic that is a synthetic derivative of doxorubicin and is considered an anthracenedione. It is believed to act by intercalating into helical double-stranded DNA causing cross links and strand breaks, thus blocking both DNA and RNA synthesis. Mitoxantrone has potent antitumor effects in vitro and has been evaluated in leukemia, lymphoma and several solid tumors in humans. Mitoxantrone also has immunosuppressive activity, inhibiting B cell, T cell and macrophage proliferation and decreasing tumor necrosis factor alpha and interleukin-2 secretion. These actions led to its evaluation in patients with progressive forms of multiple sclerosis where it was shown to have activity in decreasing the rates of relapse and development of new lesions. Mitoxantrone was approved for use in the United States in 1987 and current indications include acute non-lymphocytic leukemia and advanced prostate cancer. Mitoxantrone was subsequently approved for use in secondary progressive forms, progressive-relapsing forms and worsening relapsing-remitting forms of multiple sclerosis. Mitoxantrone is available in several generic formulations as a solution for intravenous injection (usually 2 mg/mL). Mitoxantrone is administered intravenously in doses typically ranging from 12 to 14 mg/m2 at intervals of every 3 months (multiple sclerosis) or in monthly cycles (prostate cancer and leukemia). Side effects of mitoxantrone include bone marrow suppression, nausea, vomiting, abdominal discomfort, diarrhea, alopecia, headache, dizziness and rash. Serious side effects include febrile neutropenia, cardiac toxicity [similar to that caused by doxorubicin] and secondary leukemia [in patients with multiple sclerosis]. Mitoxantrone should be administered by a physician experienced in the use of cytotoxic chemotherapy and must be given intravenously slowly and carefully, as it can cause severe local tissue damage and should not be given subcutaneously, intramuscularly or intrathecally.
Hepatotoxicity
Chemotherapy with mitoxantrone alone is associated with serum enzyme elevations in up to 40% of patients, but these elevations are generally mild-to-moderate in severity, transient and not accompanied by symptoms or jaundice. Higher rates of liver enzyme elevations have been reported with combination chemotherapeutic regimens that include mitoxantrone. In high doses, mitoxantrone has been associated with a high rate of jaundice, but the degree of hyperbilirubinemia has been mild, transient and not associated with significant serum enzyme elevations or evidence of hepatitis. Rare instances of acute liver injury have been reported in patients taking mitoxantrone, including a single case of drug-rash with eosinophilia and systemic symptoms (DRESS). The latency to onset was 8 weeks and the pattern of serum enzyme elevations was cholestatic and later mixed. Immunoallergic features were prominent and appeared to respond to corticosteroid therapy. Other drugs were being taken and the association with mitoxantrone was not definite (Case 1). Thus, idiosyncratic and clinically apparent liver injury from mitoxantrone may occur but is quite rare.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The transient ALT elevations that can occur during mitoxantrone therapy are likely due to direct toxicity of the drug or its metabolites. Idiosyncratic acute liver injury attributed to mitoxantrone is likely due to a hypersensitivity reaction. Mitoxantrone is at least partially metabolized by the liver, but the specific pathways have not been well defined.
Outcome and Management
The serum aminotransferase and mild serum bilirubin elevations that frequently accompany mitoxantrone therapy are generally self-limited, transient and unaccompanied by symptoms. The product label recommends regular monitoring of serum aminotransferase levels before each monthly or every-three-month infusion of mitoxantrone therapy, but dose modifications or discontinuation are rarely necessary. Patients who develop immunoallergic reactions to mitoxantrone should not be rechallenged with the medication. The possibility of cross reactivity with doxorubicin or other cytotoxic anthracycline antibiotics has not been evaluated, but should be done with caution.
Drug Class: Antineoplastic Agents; Multiple Sclerosis Agents
Other Drugs in the Subclass, Antibiotics, Cytotoxic: Bleomycin, Dactinomycin, Daunorubicin, Doxorubicin, Epirubicin, Idarubicin, Mitomycin, Plicamycin
CASE REPORT
Case 1. Drug reaction with eosinophilia and systemic signs (DRESS) attributed to mitoxantrone therapy.(1)
A 44 year old woman with primary, progressive multiple sclerosis developed jaundice 8 weeks after starting mitoxantrone (10 mg every 4 weeks) and piracetam (3 gm daily). She was known to have multiple sclerosis for 3 years and had been treated previously with interferon beta with little clinical improvement. At the time of her third scheduled dose of mitoxantrone, she was found to be jaundiced and had mild fever and facial edema. Laboratory testing showed a total serum bilirubin of 19.3 mg/dL which was primarily direct. Serum ALT was 561 U/L, AST 337 U/L, alkaline phosphatase 705 U/L, GGT 447 U/L. These values had been normal in the past (Table). She took no other medications except for baclofen (75 mg daily), which she had been taking for almost two years. Her medications were stopped and she was admitted for evaluation. Tests for hepatitis A, B and C were negative as were routine autoantibodies. Abdominal ultrasound and magnetic resonance imaging were normal, without evidence of biliary obstruction. However, she failed to improve and after two weeks, her serum bilirubin remained high. A liver biopsy showed canalicular cholestasis with mild portal inflammation suggestive of drug induced liver injury. One week later, she developed a generalized, erythematous, macular rash and laboratory testing demonstrated leukocytosis (15,300/μL) and eosinophilia (>4000/μL). Methylprednisone was stated (20 mg daily) and she improved rapidly. Six months after initial presentation, all liver tests were normal and she underwent drug sensitivity skin testing which showed marked reactions to mitoxantrone, but none to piracetam.
Key Points
| Medication: | Mitoxantrone (10 mg intravenously, 2 doses) |
|---|---|
| Pattern: | Cholestatic (R=1.9) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 2 months |
| Recovery: | 6 months |
| Other medications: | Piracetam, baclofen |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | Pre | Normal | Normal | Normal | |
| 8 weeks | 0 | 561 | 705 | 19.3 | Fever, facial edema |
| 10 weeks | 2 weeks | 19.9 | Liver biopsy | ||
| 11 weeks | 3 weeks | 647 | 693 | 18.1 | Rash, corticosteroids started |
| 13 weeks | 5 weeks | 8.5 | Discharged | ||
| 8 months | 6 months | Normal | Normal | Normal | Skin testing |
| Normal Values | <45 | <115 | <1.2 | ||
Comment
A woman with multiple sclerosis developed jaundice and fever 8 weeks after starting the combination of mitoxantrone and piracetam. She did not improve immediately after stopping the medications and 3 weeks later developed skin rash and eosinophilia, fully warranting a diagnosis of DRESS syndrome. A liver biopsy demonstrated canalicular cholestasis, which is typical of the intrahepatic cholestasis that is caused by medications. The immunoallergic features of DRESS syndrome improved rapidly with methylprednisolone therapy (the duration of which was unclear), and serum bilirubin began to fall. In follow up 6 months later, she had recovered completely. Skin testing suggested that mitoxantrone rather than piracetam was the cause of the hypersensitivity reaction. Piracetam is a synthetic derivative of GABA, similar in structure to levetiracetam and used as treatment of myoclonus and for cognitive impairment. It is commercially available in Europe and elsewhere, but is not approved for use in the United States. While piracetam has not been specifically linked to hypersensitivity reactions, levetiracetam has been linked to cases of DRESS and Stevens Johnson syndrome. Thus, piracetam may have been the cause of the DRESS syndrome but, without rechallenge (negative or positive), it is impossible to definitively link this outcome to either agent. Certainly, the skin test reactions favor but do not prove the contribution of mitoxantrone.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Mitoxantrone – Generic, Novantrone®
DRUG CLASS
Antineoplastic Agents; Multiple Sclerosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
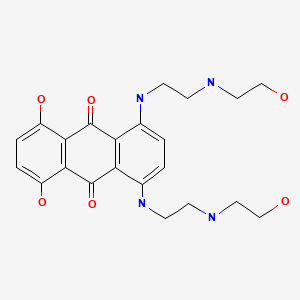
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Mitoxantrone | 70476-82-3 | C22-H28-N4-O6.2Cl-H |
|
CITED REFERENCE
- 1.
- Caruso A, Vecchio R, Patti F, Neri S. Drug rash with eosinophilia and systemic signs syndrome in a patient with multiple sclerosis. Clin Ther. 2009;31:580–4. [PubMed: 19393848]
ANNOTATED BIBLIOGRAPHY
References updated: 19 February 2020
- Zimmerman HJ. Anthracycline antibiotics. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 696-7.(Expert review of hepatotoxicity published in 1999 mentions that mitoxantrone is associated with ALT elevations in 40% of patients when used alone and in 80% when used in combination chemotherapy, but the abnormalities are usually minor and "pose no important threat").
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 553.(Review of hepatotoxicity of cancer chemotherapeutic agents mentions that mitoxantrone causes transient abnormalities in bilirubin and aminotransferase levels).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Cytotoxic agents. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1167-203.(Textbook of pharmacology and therapeutics mentions that mitoxantrone is approved for use in acute non-lymphocytic leukemia, prostate cancer and late stage, progressive multiple sclerosis).
- Paciucci PA, Sklarin NT. Mitoxantrone and hepatic toxicity. Ann Intern Med. 1986;105:805–6. [PubMed: 3767174](Among 26 patients with leukemia treated with mitoxantrone, transient ALT and AST elevations occurred in 42% and bilirubin in 4%, 4-24 days after 5 day courses of the antineoplastic agent, resolving rapidly thereafter; among 19 patients treated with mitoxantrone, vincristine and prednisone, ALT elevations occurred after 80% of courses).
- Shenkenberg TD, Von Hoff DD. Mitoxantrone: a new anticancer drug with significant clinical activity. Ann Intern Med. 1986;105:67–81. [PubMed: 3521429](Review of the biochemistry, mechanism of action, pharmacokinetics, clinical efficacy and safety of mitoxantrone in cancer chemotherapy; common side effects are mucositis, bone marrow suppression, and gastrointestinal intolerance; hepatotoxicity and ALT elevations were not mentioned).
- Sznol M, Ohnuma T, Holland JF. Hepatic toxicity of drugs used for hematologic neoplasia. Semin Liver Dis. 1987;7:237–56. [PubMed: 3317861](Overview of hepatotoxicity of antineoplastic agents, mentions that hepatotoxicity is not invariably described as a toxicity of mitoxantrone, suggesting that it is rare).
- Vredenburgh JJ, McIntyre OR, Cornwell GG 3rd, Ball ED, Cornell CJ, Mills LE, O'Donnell JF. Mitoxantrone in relapsed or refractory acute nonlymphocytic leukemia. Med Pediatr Oncol. 1988;16:187–9. [PubMed: 3380061](Among 17 patients with relapsed or refractory acute non-lymphocytic leukemia [ANLL] treated with 3 day courses of mitoxantrone, 9 [53%] developed elevated liver tests [>1.5 times ULN], but authors do not mention jaundice or clinically apparent liver injury).
- Motawy MS, Khalifa F, Salfiti R, Patel JP. Mitoxantrone, cytosine-arabinoside and 6-thioguanine (MAT) in the treatment of newly diagnosed acute non-lymphoblastic leukemia in adults. Anticancer Drugs. 1992;3:475–9. [PubMed: 1450441](Among 28 patients with newly diagnosed ANLL treated with 58 courses of mitoxantrone, cytarabine and thioguanine, major toxicities were bone marrow suppression, nausea, vomiting and stomatitis; hepatotoxicity occurred during 14 [24%] courses with Alk P elevation in all, ALT in 8 and bilirubin in 1, which resolved within an average of 12 days and did not recur upon reexposure).
- Feldman EJ, Alberts DS, Arlin Z, Ahmed T, Mittelman A, Baskind P, Peng YM, et al. Phase I clinical and pharmacokinetic evaluation of high-dose mitoxantrone in combination with cytarabine in patients with acute leukemia. J Clin Oncol. 1993;11:2002–9. [PubMed: 8410125](Study of increasing doses of mitoxantrone [40 to 80 mg/m2/day for 2 days] in combination with cytarabine in 68 patients with leukemia found severe [>3 times ULN], but reversible hyperbilirubinemia in 32% of patients, with no mention of ALT elevations or symptomatic liver injury).
- Avilés A, Guzmán R, Talavera A, García EL, Díaz-Maqueo JC. Randomized study for the treatment of adult advanced Hodgkin's disease: epirubicin, vinblastine, bleomycin, and dacarbazine (EVBD) versus mitoxantrone, vinblastine, bleomycin, and dacarbazine (MVBD). Med Pediatr Oncol. 1994;22:168–72. [PubMed: 7505877](Among 70 patients with Hodgkin disease treated with vinblastine, bleomycin and dacarbazine combined with either epirubicin or mitoxantrone, 6 of those receiving mitoxantrone, but none receiving epirubicin, developed liver test abnormalities [>3 times ULN], which became chronic in two).
- Millefiorini E, Gasperini C, Pozzilli C, D'Andrea F, Bastianello S, Trojano M, Morino S, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome. J Neurol. 1997;244:153–9. [PubMed: 9050955](Among 51 patients with relapsing multiple sclerosis enrolled in a 2 year controlled trial of mitoxantrone versus placebo, side effects included nausea, diarrhea, and amenorrhea; no mention of ALT elevations or hepatotoxicity).
- Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C, Brochet B, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997;62:112–8. [PMC free article: PMC486720] [PubMed: 9048709](Among 42 patients with active multiple sclerosis treated with once monthly, intravenous methylprednisolone [MP] alone or with mitoxantrone, side effects of mitoxantrone included amenorrhea, stomatitis, nausea and vomiting; one patient on MP alone developed "hepatitis", but no details given).
- Faggioli P, De Paschale M, Tocci A, Luoni M, Fava S, De Paoli A, Tosi A, Cassi E. Acute hepatic toxicity during cyclic chemotherapy in non-Hodgkin’s lymphoma. Haematologica. 1997;82:38–42. [PubMed: 9107080](Among 98 patients with lymphoma treated with various combinations of chemotherapy [21 received mitoxantrone], 20 had transient ALT elevations and 12 developed acute hepatitis, 8 in HBsAg positive patients and probably due to reactivation).
- Bennett JM, Young MS, Liesveld JL, Paietta E, Miller KB, Lazarus HM, Marsh RD, et al. Phase II study of combination human recombinant GM-CSF with intermediate-dose cytarabine and mitoxantrone chemotherapy in patients with high-risk myelodysplastic syndromes (RAEB, RAEBT, and CMML): an Eastern Cooperative Oncology Group Study. Am J Hematol. 2001;66:23–7. [PubMed: 11426487](Among 10 elderly patients with myelodysplastic syndromes treated with cytarabine, mitoxantrone and GM-CSF, bilirubin elevations occurred in 7 [2.2-22.4 mg/dL], but AST was raised in only 1 and alkaline phosphatase in 3 patients; 7 patients died of infectious complications).
- Schaich M, Illmer T, Aulitzky W, Bodenstein H, Clemens M, Neubauer A, Repp R, et al. SHG AML96 Study Group. Intensified double induction therapy with high dose mitoxantrone, etoposide, m-amsacrine and high dose ara-C for elderly acute myeloid leukemia patients aged 61-65 years. Haematologica. 2002;87:808–15. [PubMed: 12161356](Among 33 patients with acute myeloid leukemia treated with intensive chemotherapy with mitoxantrone, "grade 3 or 4 toxicity of the gastrointestinal tract and the liver" occurred in 48% of patients compared to only 21% of 39 patients given conventional chemotherapy).
- Hartung HP, Gonsette R, König N, Kwiecinski H, Guseo A, Morrissey SP, Krapf H, Zwingers T., Mitoxantrone in Multiple Sclerosis Study Group (MIMS). Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018–25. [PubMed: 12504397](Among 188 patients with progressive multiple sclerosis enrolled in a controlled trial of mitoxantrone vs placebo infusions every 3 months for two years, common side effects to standard doses of mitoxantrone included nausea [76%], alopecia [61%], stomatitis [19%] and diarrhea [16%]; serum ALT or AST elevations occurred in 8-9% of mitoxantrone versus 8% placebo recipients; no mention of jaundice or clinically apparent liver injury).
- Chaudhuri A, Behan PO. Mitoxantrone trial in multiple sclerosis. Lancet. 2003;361(9363):1133–4. [PubMed: 12672341](Letter in response to Hartung [2002] raising issues regarding the safety and efficacy of mitoxantrone in multiple sclerosis; no mention of hepatotoxicity).
- Cohen BA, Mikol DD. Mitoxantrone treatment of multiple sclerosis: safety considerations. Neurology. 2004;63(12) Suppl 6:S28–32. [PubMed: 15623667](Review of safety of mitoxantrone in treatment of multiple sclerosis mentions that mild-to-moderate elevations in ALT and sometimes bilirubin can occur, and recommends monitoring of routine liver tests before each infusion and deferring therapy if there are abnormalities).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, several cases were attributed to antineoplastic agents [mercaptopurine, cyclophosphamide, docetaxel, temozolomide, bortezomib and imatinib], but none to mitoxantrone).
- Caruso A, Vecchio R, Patti F, Neri S. Drug rash with eosinophilia and systemic signs syndrome in a patient with multiple sclerosis. Clin Ther. 2009;31:580–4. [PubMed: 19393848](44 year old woman with multiple sclerosis developed fever, rash and jaundice 8 weeks after starting piracetam [a neuroenhancing derivative of GABA not available in the US] and mitoxantrone [bilirubin 19.3 mg/dL, ALT 561 U/L, Alk P 705 U/L, eosinophils >4000/uL], resolving with corticosteroid therapy and later having positive skin test reactions to mitoxantrone: Case 1).
- Wundes A, Kraft GH, Bowen JD, Gooley TA, Nash RA. Mitoxantrone for worsening multiple sclerosis: tolerability, toxicity, adherence and efficacy in the clinical setting. Clin Neurol Neurosurg. 2010;112:876–82. [PubMed: 20727669](Retrospective analysis of 96 patients with multiple sclerosis treated with 534 infusions of mitoxantrone over a 6 year period at a single referral center; common side effects were nausea [64%], fatigue [53%], infections [33%], alopecia [33%], gastrointestinal upset [22%] and amenorrhea [18% of women]; no mention of ALT elevations or hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 2 attributed to antineoplastic agents [melphalan and gemtuzumab], but none to mitoxantrone).
- Kümpfel T, Gerdes LA, Flaig M, Hohlfeld R, Wollenberg A. Drug-induced Sweet's syndrome after mitoxantrone therapy in a patient with multiple sclerosis. Mult Scler. 2011;17:495–7. [PubMed: 21148263](55 year old man with multiple sclerosis developed acute febrile neutropenic dermatitis [Sweet syndrome], 7 days after a 5th three-monthly infusion of mitoxantrone which responded to stopping therapy and corticosteroids, but relapsed upon tapering dose; no mention of liver test results).
- Kim JW, Lee HJ, Yi HG, Kim BS, Bang SM, Kim JS, Kim I, et al. Mitoxantrone, etoposide, cytarabine, and melphalan (NEAM) followed by autologous stem cell transplantation for patients with chemosensitive aggressive non-Hodgkin lymphoma. Am J Hematol. 2012;87:479–83. [PubMed: 22388671](Among 69 patients with lymphoma undergoing combination chemoablation including mitoxantrone followed by HST, adverse events included mucositis [100%], febrile neutropenia [88%] and "grade 3 or 4 hepatic toxicity" in 10%, but no details given).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, ten cases were attributed to antineoplastic agents, but none to mitoxantrone).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 cases were attributed to antineoplastic agents, but none to mitoxantrone).
- Antonazzo IC, Poluzzi E, Forcesi E, Riise T, Bjornevik K, Baldin E, Muratori L, et al. Liver injury with drugs used for multiple sclerosis: contemporary analysis of the FDA Adverse Event Reporting System. Mult Scler. 2019;25:1633–40. [PubMed: 30230957](Analysis of hepatic adverse events attributed to drugs used to treat multiple sclerosis reported to the FDA between 2004 and 2016 identified 4873 cases of “severe liver injury”, most commonly due to interferon beta-1a [2343], interferon beta-1b [535], natalizumab [759], fingolimod [496], dimethyl fumarate [223], and glatiramer [212], but also mitoxantrone [131]; no specific clinical details provided).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- BLOOD COUNT IN PATIENTS WITH MULTIPLE SCLEROSIS TREATED WITH MITOXANTRONE IN SHORT TIME OBSERVATION.[Acta Pol Pharm. 2016]BLOOD COUNT IN PATIENTS WITH MULTIPLE SCLEROSIS TREATED WITH MITOXANTRONE IN SHORT TIME OBSERVATION.Pastuszak Z, Stepien A, Tomczykiewicz K, Piusinska-Macoch R. Acta Pol Pharm. 2016 Sep; 73(5):1369-1373.
- Phase I clinical and pharmacokinetic evaluation of high-dose mitoxantrone in combination with cytarabine in patients with acute leukemia.[J Clin Oncol. 1993]Phase I clinical and pharmacokinetic evaluation of high-dose mitoxantrone in combination with cytarabine in patients with acute leukemia.Feldman EJ, Alberts DS, Arlin Z, Ahmed T, Mittelman A, Baskind P, Peng YM, Baier M, Plezia P. J Clin Oncol. 1993 Oct; 11(10):2002-9.
- Review Mitoxantrone: a review of its use in multiple sclerosis.[CNS Drugs. 2004]Review Mitoxantrone: a review of its use in multiple sclerosis.Scott LJ, Figgitt DP. CNS Drugs. 2004; 18(6):379-96.
- Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer.[J Clin Oncol. 2007]Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer.Le Deley MC, Suzan F, Cutuli B, Delaloge S, Shamsaldin A, Linassier C, Clisant S, de Vathaire F, Fenaux P, Hill C. J Clin Oncol. 2007 Jan 20; 25(3):292-300. Epub 2006 Dec 11.
- Review Mitoxantrone.[Drug Intell Clin Pharm. 1986]Review Mitoxantrone.Poirier TI. Drug Intell Clin Pharm. 1986 Feb; 20(2):97-105.
- Mitoxantrone - LiverToxMitoxantrone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...