NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Levomilnacipran is a serotonin and norepinephrine reuptake inhibitor used to treat major depressive disorders. Levomilnacipran is an enantiomer of milnacipran, which is used to treat fibromyalgia. Levomilnacipran and milnacipran have been associated with a low rate of transient elevations in serum aminotransferase levels during treatment and with rare instances of clinically apparent acute liver injury with jaundice.
Background
Levomilnacipran (lee’ voe mil na’ si pran) is an oral serotonin and norepinephrine reuptake inhibitor (SNRI) that is used as a first line therapy of major depression. Levomilnacipran is the more active enantiomer of milnacipran (mil na’ si pran), which is used to treat fibromyalgia. Unlike other SNRIs such as duxoletine and venlafaxine, levomilnacipran is a more potent inhibitor of norepinephrine reuptake than serotonin reuptake. Milnacipran was approved for use in the United States in 2007 and levomilnacipran in 2013. Despite the fact that they are identical in chemical structure, the only official current indication for milnacipran is fibromyalgia, and the sole indication for levomilnacipran is the treatment of major depressive disorders. Levomilnacipran has been evaluated as therapy of fibromyalgia and phantom limb syndrome, but is not approved for those uses. Milnacripran is available in 12.5, 25, 50 and 100 mg tablets and the recommended dosage is 50 mg twice daily after a 7 day titration schedule. Levomilnacipran is available in extended release capsules of 20, 40 80 and 120 mg under the brand name Fetzima. The recommended starting dosage is 20 mg once daily, with titration upwards by 20 mg every 2 days to a maximum daily dose of 120 mg. Common side effects of milnacipran and levomilnacipran include nausea, vomiting, constipation, headache, sweating, increased heart rate, palpitations, testicular pain, urinary hesitancy and erectile dysfunction. When used with other serotonin reuptake inhibitors, milnacipran and levomilnacipran can be associated with acute serotonin syndrome of fever, tachycardia, anxiety and flushing.
Hepatotoxicity
In large clinical trials, elevations in serum aminotransferase levels during levomilnacipran and milnacipran therapy occurred in 7% of patients, but were usually mild and self-limited. Elevations above 5 times the upper limit of normal (ULN) occurred in ~1% of patients and generally resolved, even without dose adjustment or discontinuation. There has been a single published report of clinically apparent liver injury with jaundice attributed to milnacipran therapy, with a mixed pattern of liver enzyme elevations, transient increases in bilirubin without jaundice, immunoallergic features and rapid recovery on stopping (Case 1).
Likelihood score: D (possible, rare cause of clinically apparent liver injury).
Mechanism of Injury
Levomilnacipran and milnacipran are metabolized in the liver largely through the CYP 3A4 pathway and liver injury may be related to production of a toxic or immunogenic intermediate. Because of this pathway of metabolism, levomilnacipran and milnacipran are susceptible to drug-drug interactions when used with agents that strongly induce or inhibit CYP 3A4. They can also increase clotting abnormalities in patients on anticoagulants.
Outcome and Management
Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) are rare on milnacipran and levomilnacipran therapy, but should lead to dose reduction or temporary cessation. In patients with clinically apparent liver injury and jaundice, restarting therapy should be done with caution. There does not appear to be cross reactivity with other SNRIs or SSRIs and switching to another antidepressant may be the most appropriate approach. No cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome have occurred in patients receiving levomilnacipran or milnacipran.
Drug Class: Antidepressants
Other Drugs in the Subclass, SNRIs/SSRIs: Citalopram, Duloxetine, Escitalopram, Fluoxetine, Fluvoxamine, Paroxetine, Sertraline, Venlafaxine, Vilazodone, Vortioxetine
CASE REPORT
Case 1. Acute hepatitis due to milnacipran (Ixel).
[Modified from: de Widerspach-Thor A, Dubois F, Bacq Y. [Acute hepatitis associated with milnacipran treatment]. Gastroenterol Clin Biol 2004; 28: 191-2. French. PubMed Citation]
A 25 year old woman was hospitalized for epigastric pain and possible jaundice 6 months after starting milnacipran (25 mg daily) for depression. She had a past history of injection drug use and one year previously was found to have anti-HCV without HCV RNA or abnormal liver enzymes. She had been treated chronically with alprazolam for anxiety and buprenorphine for opiate addiction. She denied recent injection drug use, alcohol abuse, travel or other exposures to viral hepatitis. Over a 5 day period, she had developed epigastric pain, nausea, vomiting and headache, followed by dark urine and jaundice. On presentation, she had low grade fever, bruises on her face and cervical, axillary and inguinal lymphadenopathy. Serum ALT levels were 24 times, AST 8.5 times and alkaline phosphatase 4.3 times the upper limit of normal (Table). Serum total bilirubin was slightly above normal (1.3 mg/dL), but direct was elevated (1.0 mg/dL). The prothrombin time and differential white counts were normal. Tests for acute hepatitis A, B and C were negative (including HCV RNA), while tests for anti-HEV were conflicted, IgM anti-HEV being reactive, but IgG anti-HEV being repeatedly negative. Tests for ANA were negative and SMA minimally elevated (1:20). Abdominal ultrasound showed mild celiac adenopathy, but no evidence of biliary obstruction. Milnacipran was discontinued, but alprazolam and buprenophrine were continued. She recovered rapidly, with most enzymes falling into the near normal range within 10 days and all tests being normal 2 months later.
Key Points
| Medication: | Milnacipran (25 mg daily) |
| Pattern: | Hepatocellular (R=5.5) |
| Severity: | 1+ (liver enzyme elevations only) |
| Latency: | 6 months |
| Recovery: | Rapid, complete within 2 months |
| Other medications: | Alprazolam, buprenophrine |
Laboratory Values
* Values expressed as times the upper limit of normal were converted to absolute units.
Comment
While milnacipran has been used in Europe as an antidepressant for more than a decade, its use in the United States has been restricted to fibromyalgia. Subsequently, the levorotatory stereoisomer – levomilnacipran – was evaluated and approved for use in major depressive disorders in the United States. Both agents are associated with a low rate of serum aminotransferase elevations during therapy and only rare elevations above 3 times the upper limit of normal. This publication was the initial and, so far, only report of clinically apparent liver injury from milnacipran, and the injury described was transient and mild. The attribution of the injury to milnacipran can only be considered “probable” or “possible”, as the clinical course was also compatible with an acute viral illness or with acute hepatic necrosis (perhaps from buprenorphine or other drug overdose). On the other hand, the clinical symptoms of fever, headache, facial bruising and lymphadenopathy are also compatible with an immunoallergic hepatitis which might have been caused by milnacipran, although the long latency is atypical for immunoallergic reactions.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Levomilnacipran – Fetzima®
Milnacipran – Savella®
DRUG CLASS
Antidepressants
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Milnacipran | 101152-94-7 | C15-H22-N2-O.Cl-H |
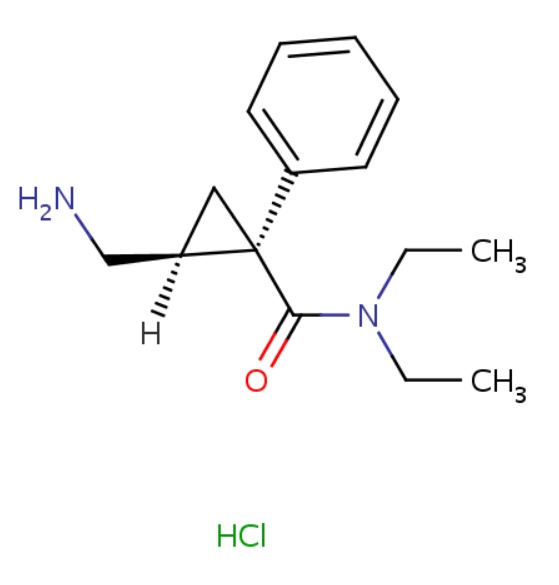 |
| Levomilnacipran | 96847-54-0 | C15-H22-N2-O |
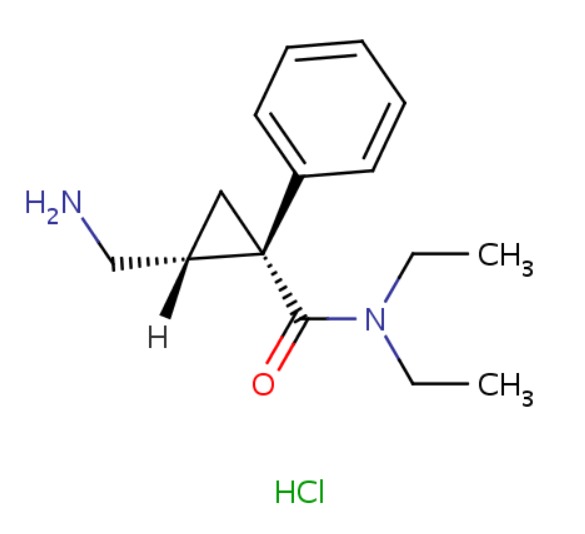 |
ANNOTATED BIBLIOGRAPHY
References updated: 20 May 2019
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of milnacipran or levomilnacipran).
- Larrey D, Ripault M-P. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 443-62.(Review of hepatotoxicity of antidepressants mentions that milnacipran has been linked to very rare cases of liver injury).
- O'Donnell JM, Shelton RC. Pharmacotherapy of depression and anxiety disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 267-78.(Textbook of pharmacology and therapeutics).
- de Widerspach-Thor A, Dubois F, Bacq Y. [Acute hepatitis associated with milnacipran treatment]. Gastroenterol Clin Biol 2004; 28: 191-2. French. [PubMed: 15060467](25 year old woman developed epigastric pain and nausea 6 months after starting milnacipran for depression and while also on buprenorphine and alprazolam [bilirubin 1.3 mg/dL, ALT ~950 U/L, Alk P ~550 U/L], resolving within 1-2 months of stopping milnacipran alone: Case 1).
- Vitton O, Gendreau M, Gendreau J, Kranzler J, Rao SG. A double-blind placebo-controlled trial of milnacipran in the treatment of fibromyalgia. Hum Psychopharmacol 2004; 19 Suppl 1: S27-35. [PubMed: 15378666](Among 125 patients with fibromyalgia in a 12 week randomized controlled trial, 7% of 86 patients taking milnacipran developed ALT or AST elevations, but all were less than twice ULN and all resolved without symptoms or bilirubin elevations).
- Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther 2008; 30: 1988-2004. [PubMed: 19108787](Among 1196 patients with fibromyalgia treated with two doses of milnacipran or placebo for 15 weeks, the most common side effects of milnacipran were nausea [34-38%], constipation [14-18%], hot flushes [11-15%], dizziness [9%] and palpitations [7-8%], and there were "no clinically relevant mean changes from baseline in any of the laboratory parameters tested").
- Mease PJ, Clauw DJ, Gendreau RM, Rao SG, Kranzler J, Chen W, Palmer RH. The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial. J Rheumatol 2009; 36: 398-409. [PubMed: 19132781](Among 888 patients with fibromyalgia treated with two doses of milnacipran or placebo for 27 weeks, the most common side effects were nausea, constipation, sweating, hot flushes, dry mouth, palpitations and hypertension, the "mean changes from baseline in laboratory values were not clinically important," and no hepatic serious adverse events were reported).
- Milnacipran (savella) for fibromyalgia. Med Lett Drugs Ther 2009; 51 (1314): 45-6. [PubMed: 19528885](Concise summary of efficacy and safety of milnacipran shortly after its approval for use in the US as therapy of fibromyalgia; mentions that milnacipran has been associated with mild ALT elevations during therapy).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to milnacipran or levomilnacipran).
- Branco JC, Zachrisson O, Perrot S, Mainguy Y; Multinational Coordinator Study Group. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in treatment of fibromyalgia. J Rheumatol 2010; 37: 851-9. [PubMed: 20156949](Among 884 patients with fibromyalgia treated with milnacipran or placebo for 17 weeks, common side effects were nausea, excessive sweating and headaches and the discontinuation rate was 22%; no mention of ALT elevations or hepatotoxicity).
- Yacoub HA, Johnson WG, Souayah N. Serotonin syndrome after administration of milnacipran for fibromyalgia. Neurology 2010; 74: 699-700. [PubMed: 20177126](76 year old man developed confusion, fever, tachycardia and hypertension 8 days after starting milnacipran and while receiving long term paroxetine; liver tests were not mentioned and he recovered upon stopping the medication).
- Levine M, Truitt CA, O'Connor AD. Cardiotoxicity and serotonin syndrome complicating a milnacipran overdose. J Med Toxicol 2011; 7: 312-6. [PMC free article: PMC3550178] [PubMed: 21735310](59 year old woman took an overdose of milnacipran [3000 mg] and developed stupor, tremor, fever, tachycardia, and respiratory failure requiring intubation, but ultimately recovered, "and liver functions were normal").
- Levomilnacipran (Fetzima) a new SNRI for depression. Med Lett Drugs Ther 2013; 55 (1432): 101-2. [PubMed: 24419243](Concise summary of clinical efficacy, safety and costs of levomilnacipran shortly after its US approval as therapy for major depression; makes no mention of ALT abnormalities).
- Mago R, Forero G, Greenberg WM, Gommoll C, Chen C. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig 2013; 33: 761-71. [PMC free article: PMC3775192] [PubMed: 23999912](Among 828 patients with major depression enrolled in a 48 week extension study of levomilnacipran, 1 patient developed transient ALT levels >3 times ULN, but without jaundice or need to discontinue treatment).
- Huskey AM, Thomas CC, Waddell JA. Occurrence of milnacipran-associated morbilliform rash and serotonin toxicity. Ann Pharmacother 2013; 47 (7-8): e32. [PubMed: 23837199](9 days after starting milnacipran and while also taking fluoxetine, doxepin and tramadol, a 57 year old woman developed full body rash, fever, restlessness, tachycardia and hypertension, the rash resolving within 4 days of stopping; no mention of liver tests results).
- Asnis GM, Bose A, Gommoll CP, Chen C, Greenberg WM. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2013 Mar; 74 (3): 242-8. [PubMed: 23561229](Among 704 patients with major depressive disorders treated for 10 weeks with one of 3 doses of levomilnacipran or placebo, mean levels of ALT and AST increased slightly in the levomilnacipran groups and 7 [1.3%] had ALT or AST elevations above 3 times ULN, although none developed jaundice).
- Scott LJ. Levomilnacipran extended-release: a review of its use in adult patients with major depressive disorder. CNS Drugs 2014; 28: 1071-82. [PubMed: 25270036](Review of 4 placebo controlled trials of levomilnacipran in 2623 patients with major depressive disorders lists the most common adverse reactions as nausea, constipation, sweating, tachycardia, palpitations, erectile dysfunction and ejaculation disorder, but does not discuss ALT elevations or hepatotoxicity).
- Shiovitz T, Greenberg WM, Chen C, Forero G, Gommoll CP. A randomized, double-blind, placebo-controlled trial of the efficacy and safety of levomilnacipran ER 40-120mg/day for prevention of relapse in patients with major depressive disorder. Innov Clin Neurosci 2014; 11: 10-22. [PMC free article: PMC3960779] [PubMed: 24653937](Among 348 patients with major depressive disorders in a 24 week continuation trial of levomilnacipran, mean levels of ALT, AST and Alk P increased, but there were no "clinically meaningful changes in liver enzymes").
- Sambunaris A, Bose A, Gommoll CP, Chen C, Greenberg WM, Sheehan DV. A phase III, double-blind, placebo-controlled, flexible-dose study of levomilnacipran extended-release in patients with major depressive disorder. J Clin Psychopharmacol 2014; 34: 47-56. [PMC free article: PMC4047313] [PubMed: 24172209](Among 434 patients with a major depressive disorder treated with 2 doses of levomilnacipran or placebo for 10 weeks, "mean changes for chemistry and hematology parameters were minor").
- Bakish D, Bose A, Gommoll C, Chen C, Nunez R, Greenberg WM, Liebowitz M, Khan A. Levomilnacipran ER 40 mg and 80 mg in patients with major depressive disorder: a phase III, randomized, double-blind, fixed-dose, placebo-controlled study. J Psychiatry Neurosci 2014; 39: 40-9. [PMC free article: PMC3868664] [PubMed: 24144196](Among 557 patients with major depressive disorders treated with one of 2 doses of levomilnacipran or placebo for 8 weeks, mean ALT, AST and Alk P levels increased slightly in the drug treated groups and 2 patients developed transient ALT levels >3 times ULN, but without jaundice or symptoms).
- Montgomery SA, Gommoll CP, Chen C, Greenberg WM. Efficacy of levomilnacipran extended-release in major depressive disorder: pooled analysis of 5 double-blind, placebo-controlled trials. CNS Spectr 2015; 20 (2): 148-56. [PMC free article: PMC4411644] [PubMed: 24902007](Among 2598 patients with major depressive disorders treated for 8-10 weeks in 5 placebo controlled trials, changes in depression rating scores were greater with levomilnacipran [-15.8] than placebo [-12.9]; no discussion of adverse events).
- Park SH, Ishino R. Liver injury associated with antidepressants. Curr Drug Saf 2013; 8: 207-23. [PubMed: 23914755](Review of hepatotoxicity of modern antidepressants, mentions that Milnacipran is a SNRI but approved in the US only for fibromyalgia and that it has not been linked to instances of liver injury in the published literature).
- Deardorff WJ, Grossberg GT. A review of the clinical efficacy, safety and tolerability of the antidepressants vilazodone, levomilnacipran and vortioxetine. Expert Opin Pharmacother 2014; 15: 2525-42. [PubMed: 25224953](Review of the safety and efficacy of levomilnacipran mentions the most frequent adverse events were nausea, vomiting, constipation, sweating, erectile dysfunction, tachycardia and palpitations; no mention of ALT elevations or hepatotoxicity).
- Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry 2014; 171: 404-15. [PubMed: 24362450](Review of hepatotoxicity of antidepressants, does not discuss milnacipran or levomilnacipran).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. PubMed Citation. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 20 cases [2%] were attributed to antidepressants including 9 due to SNRIs including duloxetine [n=7], nefazodone [n=1], trazodone [n=1], and 5 to SSRIs including escitalopram [n=3], fluoxetine [n=1] and sertraline [n=1], but none were attributed to milnacipran or levomilnacipran).
- Friedrich ME, Akimova E, Huf W, Konstantinidis A, Papageorgiou K, Winkler D, Toto S, et al. Drug-induced liver injury during antidepressant treatment: results of AMSP, a drug surveillance program. Int J Neuropsychopharmacol 2016; 19. pii: pyv126. PubMed Citation. [PMC free article: PMC4851269] [PubMed: 26721950](Among 184,234 psychiatric inpatients from 80 hospitals, 149 cases [0.08%] of drug induced liver injury were reported, but none were atributed to milnacipran or levomilnacipran).
- Ferrajolo C, Scavone C, Donati M, Bortolami O, Stoppa G, Motola D, Vannacci A, et al.; DILI-IT Study Group. Antidepressant-induced acute liver injury: a case-control study in an Italian inpatient population. Drug Saf 2018; 41: 95-102. PubMed Citation. [PubMed: 28770534](Among 179 cases of hospitalizations for unexplained acute liver injury enrolled in an Italian prospective study between 2010 and 2014, 17 had been exposed to an antidepressant including 4 to citalopram, 3 sertraline, 3 amitriptyline and 2 paroxetine, but none were linked to milnacipran or levomilnacipran).
- Billioti de Gage S, Collin C, Le-Tri T, Pariente A, Bégaud B, Verdoux H, Dray-Spira R, et al. Antidepressants and hepatotoxicity: A cohort study among 5 million individuals registered in the French National Health Insurance Database. CNS Drugs 2018; 32: 673-84. (Among 5 million persons identified in a national French health insurance database who started an antidepressant between 2010 and 2015, 382 developed serious liver injury resulting in hospitalization, rates per 100,0000 persons-years being 19 for SSRIs, 22 venlafaxine, 13 duloxetine, and 33 mirtazapine while among 37,577 persons who started milnacipran, there were no hospitalizations for liver injury). [PMC free article: PMC6061298] [PubMed: 29959758]
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Levomilnacipran (Fetzima): A New Serotonin-Norepinephrine Reuptake Inhibitor for the Treatment of Major Depressive Disorder.[J Pharm Pract. 2014]Review Levomilnacipran (Fetzima): A New Serotonin-Norepinephrine Reuptake Inhibitor for the Treatment of Major Depressive Disorder.Saraceni MM, Venci JV, Gandhi MA. J Pharm Pract. 2014 Aug; 27(4):389-95. Epub 2013 Dec 31.
- Review The Role of Levomilnacipran in the Management of Major Depressive Disorder: A Comprehensive Review.[Curr Neuropharmacol. 2016]Review The Role of Levomilnacipran in the Management of Major Depressive Disorder: A Comprehensive Review.Bruno A, Morabito P, Spina E, Muscatello MR. Curr Neuropharmacol. 2016; 14(2):191-9.
- Levomilnacipran Pharmacokinetics in Healthy Volunteers Versus Patients with Major Depressive Disorder and Implications for Norepinephrine and Serotonin Reuptake Inhibition.[Clin Ther. 2015]Levomilnacipran Pharmacokinetics in Healthy Volunteers Versus Patients with Major Depressive Disorder and Implications for Norepinephrine and Serotonin Reuptake Inhibition.Chen L, Greenberg WM, Gommoll C, O'Connor J, Zukin SR, Periclou A, Ghahramani P. Clin Ther. 2015 Sep; 37(9):2059-70. Epub 2015 Aug 20.
- Review Levomilnacipran extended-release: a review of its use in adult patients with major depressive disorder.[CNS Drugs. 2014]Review Levomilnacipran extended-release: a review of its use in adult patients with major depressive disorder.Scott LJ. CNS Drugs. 2014 Nov; 28(11):1071-82.
- Serotonin and Norepinephrine Reuptake Inhibitors.[Handb Exp Pharmacol. 2019]Serotonin and Norepinephrine Reuptake Inhibitors.Shelton RC. Handb Exp Pharmacol. 2019; 250:145-180.
- Milnacipran - LiverToxMilnacipran - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...