NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Methyldopa (alpha-methyldopa or α-methyldopa) is a centrally active sympatholytic agent that has been used for more than 50 years for the treatment of hypertension. Methyldopa has been clearly linked to instances of acute and chronic liver injury that can be severe and even fatal.
Background
Methyldopa (meth" il doe' pa) is a centrally active sympatholytic agent that reduces sympathic drive to the heart and peripheral circulation, leading to decreased cardiac output and lowered peripheral arterial resistance. Introduced in 1960, methyldopa rapidly became a leading antihypertensive agent, but in the last two decades its use has decreased markedly, replaced by better tolerated and more effective antihypertensive medications. Currently, the major use of methyldopa is treatment of hypertension during pregnancy, a use based upon its established record of safety during pregnancy and breast feeding. Methyldopa is available generically and formerly under the trade name Aldomet as 125, 250 and 500 mg tablets. Fixed combinations with hydrochlorothiazide are also available (Aldoril). The recommended maintenance dose in adults is 500 mg to 2 g daily in 2-4 divided doses. Common side effects include nausea, diarrhea, headache, dizziness, sedation, dry mouth and rash. Rare but potentially severe adverse effects include hemolytic anemia (Coombs positive), lupus-like syndrome, mycocarditis, pancreatitis and hepatotoxicity.
Hepatotoxicity
Drug induced liver injury due to methyldopa was identified shortly after its introduction into medical use in the 1960’s. Chronic use of methyldopa is associated with mild and transient elevations in serum aminotransferase levels in 5% to 35% of patients, these elevations often resolving despite continuation of the medication. In contrast, clinically apparent or significant liver injury from methyldopa is relatively uncommon, although several hundred cases have been reported. Two patterns of hepatotoxicity have been described: an acute hepatitis that appears within weeks to months of starting treatment, and a chronic hepatitis that arises months to years after initiation of methyldopa therapy.
The acute liver injury from methyldopa generally arises within 2 to 12 weeks of starting therapy and is typically hepatocellular with marked elevations in ALT and AST (5- to 100-fold) and modest increases in alkaline phosphatase, although in a small proportion of patients the pattern of enzyme elevations is mixed or cholestatic (Case 1 and 2). Most patients become jaundiced. Symptoms resemble those of acute viral hepatitis, including fever, headache, fatigue, anorexia and nausea. Signs of hypersensitivity other than fever are uncommon. The injury can be severe and fatal. While some cases are associated with marked cholestasis and prolonged jaundice, most patients recover within 4 to 12 weeks. Autoantibodies including Coombs and antinuclear antibody positivity may be present (but also can arise independent of liver injury). Liver biopsy shows an acute hepatitis-like picture with marked inflammatory infiltrates and fatty change, with variable amounts of necrosis. Rechallenge leads to rapid recurrence of liver injury and can result in severe hepatitis, acute liver failure and death.
The chronic liver injury from methyldopa usually arises after 6 months, but may become first evident after several years of therapy (Case 3). This chronic hepatitis-like clinical picture has a more insidious onset typically with fatigue, weakness and nausea associated with mild or no jaundice. Clinical features may include liver enlargement and tenderness and spider angiomata. The clinical and laboratory pattern often resembles autoimmune hepatitis, with moderate to marked elevations in ALT and AST, modest alkaline phosphatase elevations, increases in immunoglobulin levels (particularly IgG), and high titers of autoantibodies such as antinuclear antibody (ANA) and smooth muscle antibody (SMA). Liver biopsy demonstrates findings of chronic active hepatitis with variable amounts of fatty change and fibrosis. Plasma cell infiltrates may be prominent. Cirrhosis and end stage liver disease can occur if the drug is continued. The disease resolves slowly but completely with discontinuation of methyldopa. Chronic liver injury now appears to be the most common form of drug induced liver injury from this agent. Some cases of methyldopa induced liver injury have features of both acute and chronic injury and the two forms of hepatic injury may share a common etiology.
African Americans appear to have a higher risk for liver injury from methyldopa than Caucasians or Hispanic individuals. The course may be more severe and outcome less favorable in Africans Americans as well. Granulomatous hepatitis can also occur with methyldopa therapy, usually in association with drug fever and systemic symptoms (and granulomas elsewhere), and sometimes with granulomatous myocarditis which can be fatal. In these situations, the liver injury is usually mild and anicteric.
Likelihood score: A (well known cause of clinically apparent liver injury).
Mechanism of Hepatotoxicity
Both the acute and chronic hepatic injury from methyldopa have features that suggest an immune etiology, although less allergic than autoimmune in character. These findings and metabolic studies suggest that methyldopa may induce an autoimmune liver injury (perhaps via a toxic metabolic intermediate serving as an antigenic hapten presented on the surface of hepatocytes) in susceptible hosts.
Outcome and Management
Both the acute and the chronic forms of liver injury from methyldopa can be severe, particularly if the medication is continued despite appearance of clinically significant injury. Recovery usually occurs within 6 to 8 weeks, but patients with chronic hepatitis can be left with inactive cirrhosis. Methyldopa ranks as one of the ten most common causes of acute liver failure due to medications, although its frequency is decreasing as its use has become more restricted. Because of its cost, methyldopa is still used widely for treatment of hypertension in developing nations, where cases of liver injury are likely to continue to arise. Patients with methyldopa induced liver injury should not be reexposed to this medication, but there is no evidence that there is cross susceptibility to liver injury with other antihypertensive agents. Prednisone has been used to treat both the acute and the chronic injury from methyldopa with unclear benefit. Management should focus on early withdrawal of methyldopa, and treatment with corticosteroids should be restricted only to severe or persistent cases and withdrawn in a timely manner.
Drug Class: Antihypertensive Agents
CASE REPORTS
Case 1. Abnormal serum aminotransferase levels developing during methyldopa therapy.(1)
A 29 year old woman was found to have hypertension during the first trimester of her first pregnancy and was started on methyldopa in a dose of 500 mg twice daily. Eight weeks later, during a routine prenatal visit, she was found to have elevations in serum aminotransferase levels. She was without symptoms of liver disease and denied all previous history of hepatitis or jaundice and any exposures or high risk behaviors. Serum alkaline phosphatase levels were minimally elevated and bilirubin, albumin and prothrombin time were normal. An abdominal ultrasound showed no abnormality of the liver or bile ducts. Serum ANA was positive in a titer of 1:160. She also had equivocal tests for anti-HCV and VDRL, both of which were later shown to be false positives. Methyldopa was stopped and her liver tests were normal one month later.
Key Points
| Medication: | Methyldopa |
|---|---|
| Pattern: | Hepatocellular (R=28) |
| Severity: | 1+ (no jaundice) |
| Latency: | 8 weeks |
| Recovery: | Complete within 1 month |
| Other medications: | Nifedipine, multivitamins |
Laboratory Values
Comment
This case is typical of asymptomatic elevations in serum aminotransferase levels that can occur during methyldopa therapy. These were indentified on routine testing and not as a result of symptoms or specific monitoring for hepatotoxicity. Recovery was rapid. The co-occurrence of ANA positivity and false positive VDRL and anti-HCV reactivity was probably due to methyldopa induced immune activation and hyperglobulinemia.
Case 2. Acute hepatitis due to methyldopa.(2)
A 55 year old woman had been treated for hypertension intermittently with methyldopa in the past and then developed jaundice and fatigue 7 days after it was restarted. She had no other significant past medical history, took no other medications, did not drink alcohol and had no risk factors for viral hepatitis or liver disease. On admission, she was jaundiced and serum bilirubin was 6.8 mg/dL (5.0 mg/dL direct), AST 858 U/L and alkaline phosphatase 214 U/L (Table). A liver biopsy was compatible with acute drug induced liver injury. Markers of hepatitis B were negative. Further study showed that she had elevations in IgG, IgA and IgM, and was positive for smooth muscle (SMA) and antinuclear (ANA) antibodies and had a positive direct Coombs test. She was not anemic. Methyldopa was stopped and she recovered clinically quite rapidly; but biochemical and immunological abnormalities resolved only slowly over the next few months. Ultimately most abnormalities fell to normal or near normal, although SMA persisted in unchanging titers.
Key Points
| Medication: | Methyldopa |
|---|---|
| Pattern: | Hepatocellular (R=8.6) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 1 week (prior exposure) |
| Recovery: | Complete by 6 months |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Methyldopa, which had been used intermittently for at least a year, was given for 7 days | |||||
| 1 week | 0 | 858 | 214 | 6.8 | |
| 1 month | 300 | 140 | 1.6 | SMA+, ANA+, Coombs+ | |
| 3 months | 100 | 95 | 0.8 | IgG 2300, IgA 600, IgM 300 mg/dL | |
| 5 months | 78 | 09 | SMA+, ANA-, Coombs- | ||
| 6 months | 63 | 88 | |||
| 7 months | 47 | 90 | IgG 1300, IgA 250, IgM 78 mg/dL | ||
| Normal Values | <40 | <86 | <1.2 | ||
Comment
Although the latency period was very short, this case was otherwise typical of the acute hepatocellular injury caused by methyldopa. Aminotransferase levels were more than 20-fold elevated while alkaline phosphatase was minimally increased. Autoantibodies and hypergammaglobulinemia can develop with methyldopa induced liver disease and give a clinical picture that resembles an acute onset of autoimmune hepatitis. However, in this case, the disease improved with discontinuation of methyldopa and the autoantibodies and elevated immunoglobulin levels ultimately improved once the liver injury had settled. Nevertheless, the minor abnormalities of serum enzymes many months after the injury are a good reason to continue to follow the patient for evidence of an underlying liver disease. She should be cautioned against receiving methyldopa again.
Case 3. Chronic hepatitis caused by long term methyldopa therapy.(1)
A 25 year old woman developed signs and symptoms of chronic liver disease after 8 months of therapy with methyldopa. Methyldopa had been started in a dose of 250 mg twice daily during a pregnancy, but was then continued after she had a Caesarian section 3 months later. After being on methyldopa for 8 months, she had the insidious onset of nausea, dark urine, itching and jaundice. She was admitted to a local hospital and laboratory testing showed an ALT of 1292 U/L and bilirubin of 7.3 mg/dL. Tests for hepatitis A, B and C were negative. Both smooth muscle and antinuclear antibody were negative. CT scans and ultrasound of the liver were normal. A liver biopsy showed changes typical of chronic active hepatitis. Methyldopa was stopped, and she was placed on prednisone. Serum aminotransferases slowly improved. Six months later prednisone was stopped and in follow up her liver tests remained normal.
Key Points
| Medication: | Methyldopa |
|---|---|
| Pattern: | Hepatocellular (R=21) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 8 months |
| Recovery: | Complete after 6 month course of prednisone |
| Other medications: | Triamterene |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Methyldopa started during pregnancy | |||||
| 8 months | 0 | 1292 | 126 | 10.3 | Methyldopa stopped |
| 1 week | 1053 | 101 | 19.3 | ||
| 2 weeks | 1140 | 156 | 24.9 | Prednisone started | |
| 9 months | 4 weeks | 362 | 169 | 11.9 | |
| 6 weeks | 88 | 102 | 3.0 | ||
| 10 months | 8 weeks | 64 | 113 | 2.0 | |
| 11 months | 3 months | 80 | 61 | 1.0 | Prednisone tapered |
| 12 months | 4 months | 45 | 74 | 1.0 | |
| 14 months | 6 months | 29 | 69 | 0.5 | Prednisone stopped |
| 20 months | 12 months | 19 | 83 | 1.0 | |
| Normal Values | <60 | <126 | <1.2 | ||
Comment
This case represents an example of severe chronic active hepatitis induced by methyldopa. The use of prednisone is controversial, but the height of the bilirubin and ALT elevation led to its use. Importantly, once jaundice had resolved, the prednisone was withdrawn gradually and, in follow up, this patient was asymptomatic and had normal liver tests. Many cases of methyldopa induced acute and chronic hepatitis are accompanied by high levels of autoantibodies and immunoglobulin elevations. Comparison of cases with and without these autoimmune features, however, show little difference in clinical features, severity of injury, hepatic histology or outcome, suggesting that they are similarly immune mediated and that the autoantibodies do not play a pathogenetic role.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Methyldopa – Generic, Aldomet® (Currently discontinued)
DRUG CLASS
Antihypertensive Agents
Product labeling at DailyMed, National Library of Medicine, NIH
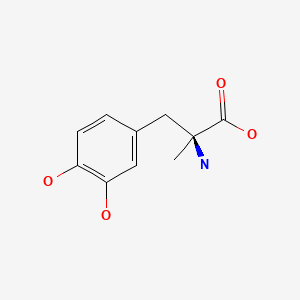
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Methyldopa | 555-30-6 | C10-H13-N-O4 |
|
CITED REFERENCES
- 1.
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056]
- 2.
- Delpre G, Grinblat J, Kadish U, Livni E, Shoha B. Case report. Immunological studies in a case of hepatitis following methyldopa administration. Am J Med Sci 1979; 277: 207-13. PMID:37733. [PubMed: 37733]
ANNOTATED BIBLIOGRAPHY
References updated: 15 January 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd edition. Philadelphia: Lippincott Williams & Wilkins, 1999. pp. 656-8.(Expert review of methyldopa induced liver injury from 1999. At least 150 instances of hepatoxicity from methyldopa have been described; usually a hepatocellular pattern of injury and can present as a chronic hepatitis; rash and esoinophilia are rare, but ANA is often present).
- De Marzio DH, Navarro VJ. Antihypertensives. Hepatotoxicity of cardiovascular and antidiabetic drugs: antihypertensives. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 522-5.(Review of hepatotoxicity of antihypertensive agents mentions that hepatotoxicity from methyldopa resembles acute viral hepatitis and the toxicity appears to be immune mediated).
- Eschenhagen T. Treatment of hypertension. In, Brunton LL, Hillal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 507-26.(Textbook of pharmacology and therapeutics).
- Gillespie L Jr, Oates JA, Crout JR, Sjoerdsma A. Clinical and chemical studies with alpha-methyl-dopa in patients with hypertension. Circulation. 1962;25:281–91. [PubMed: 13898638](Review of 80 patients treated with methyldopa; 2 developed fever with accompanying abnormal AST and bilirubin levels, with rapid resolution upon stopping).
- Williams ER, Khan MA. Liver damage in patients on methyldopa. J Ther Clin Res. 1967;1:5–7. Not in PubMed.(Summarized in Tysell et al. [1971]: two cases of liver injury appearing after 2 and 18 months of methyldopa therapy, with hepatocellular injury and jaundice).
- Zarday Z, Rosenthal WS, Wolff FW. Severe liver toxicity after methyldopa. N Y State J Med. 1967;67:1897–9. [PubMed: 5232672](37 year old man developed jaundice 2-3 months after starting methyldopa [bilirubin rising to 30.3 mg/dL, AST 1,120 U/L, Alk P 4 times ULN], worsening for 2 weeks after stopping before gradually resolving).
- Elkington SG, Schreiber WM, Conn HO. Hepatic injury caused by L-alpha-methyldopa. Circulation. 1969;40:589–95. [PubMed: 5823554](37 year old man developed symptoms within 2 weeks of starting methyldopa, but it was continued for 4 months [biliubin 14.0 mg/dL, AST 1410 U/L, Alk P 3 times ULN], resolving after stopping and recurring in response to two rechallenges, but only after several weeks of exposure).
- Eliastam M, Holmes AW. Hepatitis, arthritis and lupus cell phenomena caused by methyldopa. Am J Dig Dis. 1971;16:1014–8. [PubMed: 4108488](40 year old man developed jaundice 2 months after starting methyldopa [bilirubin 7.4 mg/dL, ALT 1000 U/L, Alk P 2 times ULN] that began to resolve, but rapidly recurred upon reexposure for 2 weeks [bilirubin 5.1 mg/dL, ALT 1200 U/L, Alk P 2 times ULN], ultimately resolving).
- Tysell JE Jr, Knauer M. Hepatitis induced by methyldopa (aldomet). Report of a case and a review of the literature. Am J Dig Dis. 1971;16:848–55. [PubMed: 5098212](38 year old woman developed jaundice 2 months after starting methyldopa [bilirubin 6.1 mg/dL, ALT 1300 U/L, Alk P 3 times ULN], resolving spontaneously and recurring more rapidly [19 days] and severely [bilirubin 8.5 mg/dL, ALT 1240 U/L, Alk P 2 times ULN] on reexposure 3 years later).
- Wong ML. Hepatocellular damage due to methyldopa. Med J Malaya. 1971;25:218–9. [PubMed: 4253251]
- Brouillard RP, Barret O Jr. Methyldopa associated hepatitis. JAMA. 1973;224:904. [PubMed: 4739696](56 year old woman developed jaundice 41 days after starting methyldopa [bilirubin 15.0 mg/dL, AST 2070 U/L], recovering within 2 months of stopping).
- Goldstein GB, Lam KC, Mistilis SP. Drug-induced active chronic hepatitis. Am J Dig Dis. 1973;18:177–84. [PubMed: 4688569](Among 21 cases of "active chronic hepatitis" presenting over a 1 year period, 9 were due to oxyphenisatin, 5 to methyldopa and 9 were idiopathic; methyldopa cases were all jaundiced and AST 200-600 U/L, latency averaged 15 months, all responded to corticosteroids; no mention of ANA).
- Hoyumpa AM Jr, Connell AM. Methyldopa hepatitis. Report of three cases. Am J Dig Dis. 1973;18:213–22. [PubMed: 4688573](Three women, ages 50-55 years, developed jaundice and acute hepatitis within 8-10 weeks of starting methyldopa, one case fatal and another protracted; severe recurrence with reexposure).
- Rehman OU, Keith TA, Gall EA. Methyldopa-induced submassive hepatic necrosis. JAMA. 1973;224:1390–2. [PubMed: 4739987](51 year old woman developed jaundice 3 months after starting methyldopa with recovery, but fatal recurrence upon reexposure).
- Torres Gomez JM. Intrahepatic cholestasis due to alpha-methyldopa: a case report. Bol Asoc Med P R. 1973;65:212–4. [PubMed: 4531928]
- Hoffbrand BI, Fry W, Bunton GL. Cholestatic jaundice due to methyldopa. Br Med J. 1974;3:559. [PMC free article: PMC1611506] [PubMed: 4412424](51 year old woman developed jaundice and pruritus 2 months after starting methyldopa [bilirubin 11.3 mg/dL, AST and ALk P 2 times ULN, ANA negative], resolving slowly over 3 months after stopping).
- Schweitzer IL, Peters RL. Acute submassive hepatic necrosis due to methyldopa. A case demonstrating possible initiation of chronic liver disease. Gastroenterology. 1974;66:1203–11. [PubMed: 4133500](49 year old woman developed severe acute hepatitis 10 weeks after starting methyldopa [bilirubin 22.1 mg/dL, ALT 1860 U/L, Alk P 3 times ULN], with biopsy findings suggesting chronicity and Coombs and LE prep positivity; positive rechallenge [ALT 500 U/L after 8 days of reexposure] with 2 more biopsies).
- Toghill PJ, Smith PG, Benton P, Brown RC, Matthews HL. Methyldopa liver damage. Br Med J. 1974;3:545–8. [PMC free article: PMC1611511] [PubMed: 4414663](Characterization of 20 cases of methyldopa induced liver injury; latency 2-32 weeks, most <6 weeks, all jaundiced, mostly hepatocellular or mixed, but two cholestatic, resolved with stopping; liver biopsies showing chronic active hepatitis in 2, acute liver failure in 2, cirrhosis in 2; severe recurrences with reexposure).
- Toghill PJ, Smith PG, Benton P, Brown RC, Matthews HL. Proceedings: Liver damage in patients taking methyldopa. Gut. 1974;15:342–3. [PubMed: 4834576](Abstract summarizing 20 cases of methyldopa hepatotoxicity described in Toghill [1974]).
- Maddrey WC, Boitnott JK. Severe hepatitis from methyldopa. Gastroenterology. 1975;68:351–360. [PubMed: 22550758](6 cases of methyldopa hepatotoxicity in 2 year period, all women, ages 38-62 years, onset of symptoms in 1-2 weeks, jaundice after 2-6 weeks; bilirubin 11.8-29.4 mg/dL, ALT 122-710 U/L, Alk P < twice ULN; 1 died; 2 had rash).
- Sataline L, Lowell D. Delayed hepatotoxicity from methyldopa. Conn Med. 1975;39:775–6. [PubMed: 1204341](39 year old man developed acute hepatitis 3 years after starting methyldopa [bilirubin 6.8 mg/dL, AST 180 U/L, Alk P 188 U/L], resolving within 2 months of stopping).
- Bonkowsky HL, Brisbane J. Colitis and hepatitis caused by methyldopa. JAMA. 1976;236:1602–3. [PubMed: 989134](55 year old man developed fever, rash, eosinophilia [11%], and diarrhea within 10 days of starting methyldopa [bilirubin 2.3 mg/dL, AST 150 U/L, Alk P 181 U/L], rapid improvement upon stopping, but immediate recurrence with single dose rechallenge).
- Miller AC Jr, Reid WM. Methyldopa-induced granulomatous hepatitis. JAMA. 1976;235:2001–2. [PubMed: 946514](49 year old woman developed fever, myalgias and nausea within 2 days of starting methyldopa, minimal AST and Alk P elevations; liver biopsy showed granulomas; not so much hepatotoxicity as drug-fever, with systemic granulomas).
- Rodman JS, Deutsch DJ, Gutman SI. Methyldopa Hepatitis. A report of six cases and review of the literature. Am J Med. 1976;60:941–8. [PubMed: 937354](Six cases of methyldopa hepatotoxicity, including 4 women and 2 men, 2 fatal, usually hepatocellular with latency of 4-12 weeks, resolution in 4-12 weeks, occasionally with Coombs positivity; review of 77 additional cases from the literature).
- Thomas E. Methyldopa liver injury. J Assoc Physicians India. 1976;24:851–3. [PubMed: 1028823]
- Thomas E, Bhuta S, Rosenthal WS. Methyldopa-induced liver injury. Rapid progression to fatal postnecrotic cirrhosis. Arch Pathol Lab Med. 1976;100:132–5. [PubMed: 946400](55 year old woman developed severe hepatitis 12 weeks after starting methyldopa [bilirubin 13.9 mg/dL, AST 900 U/L, Alk P 150 U/L], treated with corticosteroids, but developed subacute liver failure and death, not actually cirrhosis).
- Puppala AR, Steinheber FU. Fulminant hepatic failure associated with methyldopa. Am J Gastroenterol 1977; 68: 578-81. PMID: 77129. [PubMed: 77129](66 year old woman developed acute liver failure after 6 months of intermittent therapy with methyldopa, LE prepation positive, ANA negative).
- Sakamaki H, Dan K, Onozawa Y, Adachi Y, Ukishima H. [A case of alpha-methyldopa-induced hemolytic anemia with cholestasis (author's transl)] Rinsho Ketsueki 1977; 18: 821-7. Japanese. PMID: 916211. [PubMed: 916211]
- Sotaniemi EA, Hokkanen OT, Ahokas JT, Pelkonen RO, Ahlqvist J. Hepatic injury and drug metabolism in patients with alpha-methyldopa-induced liver damage. Eur J Clin Pharmacol. 1977;12:429–35. [PubMed: 598417](Summary of 36 cases of methyldopa hepatotoxicity, 14 with acute presentation with hepatocellular or mixed injury and jaundice within 1-6 months and 22 with chronic onset, often insidiuous, injury usually mixed and anicteric arising within 12-24 months of starting; 4 cases occurred in one family).
- Thomas E, Rosenthal WS, Zapiach L, Micci D. Spectrum of methyldopa liver injury. Am J Gastroenterol. 1977;68:125–33. [PubMed: 920711](Seven cases of methyldopa hepatotoxicity with hepatocellular injury and jaundice occurring 6-12 weeks after starting methyldopa, only one with autoantibodies, one fatal, all jaundiced).
- Furhoff AK. Adverse reactions with methyldopa--a decade's reports. Acta Med Scand. 1978;203:425–8. [PubMed: 149490](Summary of 75 Swedish adverse event reports on methyldopa between 1966-75; fever in 166 [latency usually <3 weeks], hemolysis 67 [2 months to years], liver injury 29 [1 month to years], allergic reactions 23, gastrointestinal 17, psychiatric 13, other 27; those with fever often had mild ALT elevations).
- Hokkanen OT, Sotaniemi EA. Liver injury and multiple drug therapy. Arch Toxicol Suppl. 1978;(1):173–6. [PubMed: 277098](Description of 100 cases of drug induced liver injury presenting over 10 year period to one Finnish center; 36 due to sulphonamides, 16 nitrofurantoin, 20 contraceptives, 10 methyldopa [10%]).
- Delpre G, Grinblat J, Kadish U, Livni E, Shoha B. Case report. Immunological studies in a case of hepatitis following methyldopa administration. Am J Med Sci 1979; 277: 207-13. PMID: 37733. [PubMed: 37733](55 year old woman developed acute hepatitis 7 days after starting methyldopa [bilirubin 6.8 mg/dL, AST 858 U/L, Alk P 214 U/L], with concurrent autoantibodies, ANA disappeared after stopping but SMA remained present; extensive immunologic tests also performed).
- Seggie J, Saunders SJ, Kirsch RE, Campbell JAH, Gitlin N, Clain D, Terblanche J. Patterns of hepatic injury induced by methyldopa. S Afr Med J. 1979;55:75–83. [PubMed: 424937](12 patients with methyldopa hepatotoxicity, 9 with acute hepatocellular disease and jaundice with onset in 1-9 weeks, of whom 2 died; 3 patients developed chronic disease arising after 1-7 years of therapy accompanied by mild jaundice and ALT elevations, one not fully resolving by 8 months after stopping).
- Shashaty GG. Cryptogenic cirrhosis associated with methyldopa. South Med J. 1979;72:364–6. [PubMed: 424836](55 year old woman presented with ascites and cryptogenic cirrhosis having been on methyldopa for 5 years, with normal ALT, AST and bilirubin; Coombs positive).
- Beaugrand M, Gavillon C, Ferrier JP. [High levels of endoplasmic reticulum antibody titer in a case of alpha-methyldopa-induced chronic active hepatitis (author's transl)] Gastroenterol Clin Biol 1980; 4: 219-21. French. PMID: 7380145. [PubMed: 7380145]
- Arranto AJ, Sotaniemi EA. Morphologic alterations in patients with alpha-methyldopa-induced liver damage after short- and long-term exposure. Scand J Gastroenterol. 1981;16:853–63. [PubMed: 7323715](Comparison of 7 patients with acute icteric hepatitis after short term [3-6 months] and 24 with chronic usually anicteric hepatitis after long term [3-11 years] methyldopa; histology showed fat and some fibrosis in chronic cases, but also showed chronic hepatitis).
- Arranto AJ, Sotaniemi EA. Histologic follow-up of alpha-methyldopa-induced liver injury. Scand J Gastroenterol. 1981;16:865–72. [PubMed: 7323716](Follow up liver biopsies in 6 patients with chronic methyldopa injury, 7-24 months later; resolution of enzyme elevations and clinical improvement occurred, but liver biopsies showed persistence of of fat and fibrosis, one patient developed cirrhosis).
- Balázs M, Kovách G. Chronic aggressive hepatitis after methyldopa treatment. Case report with electron-microscopic study. Hepatogastroenterology. 1981;28:199–202. [PubMed: 7274982](52 year old woman developed liver injury 4 months after starting methyldopa, which resolved rapidly upon stopping and recurred within 3 weeks of reexposure [bilirubin 0.9 mg/dL, ALT 354 U/L, Alk P 2 times ULN]; biopsy suggested chronic active hepatitis).
- Bezahler GH. Fatal methyldopa-associated granulomatous hepatitis and myocarditis. Am J Med Sci. 1982;283:41–5. [PubMed: 7055158](78 year old woman developed drug fever after years of methyldopa therapy, sudden death and autopsy showed granulomatous myocarditis, many granulomas in liver and elsewhere, minimal ALT elevation and no jaundice, indicative of drug fever with systemic granulomas).
- Breland BD, Hicks GS Jr. Hepatitis and hemolytic anemia associated with methyldopa therapy. Drug Intell Clin Pharm. 1982;16:489–92. [PubMed: 7094845](56 year old man presented with jaundice after 7 years of methyldopa therapy [bilirubin 41 mg/dL, AST 105 U/L, Alk P 122 U/L, ANA negative, Coombs positive]; liver biopsy showed cirrhosis with slow and incomplete recovery and associated hemolytic anemia that resolved more rapidly upon stopping).
- Dossing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol. 1982;17:205–11. [PubMed: 6982502](Review of 572 Danish cases of drug induced liver injury between 1968-78, methyldopa accounted for 11 cases, 8 with acute and 3 with chronic presentations).
- Seeverens H, de Bruin CD, Jordans JG. Myocarditis and methyldopa. Acta Med Scand. 1982;211:233–5. [PubMed: 7080870](Autopsy series of 6 patients who died suddenly due to granulomatous myocarditis while receiving methyldopa [for 16 days to 3 years], most also had granulomas in liver or chronic hepatitis; no clinical information).
- Orozco López P, Romá¡n Martínez J, Sorribes Puelles R, Trilla Soler M, Pujol Fernández C. [Carbamazepine and methyldopa: a hepatotoxic combination?] Med Clin (Barc) 1983; 81: 40-1. Spanish. PMID: 6888059. [PubMed: 6888059]
- Shalev O, Mosseri M, Ariel I, Stalnikowicz R. Methyldopa-induced immune hemolytic anemia and chronic active hepatitis. Arch Intern Med. 1983;143:592–3. [PubMed: 6830396](76 year old man developed mild hepatitis [bilirubin 2.1 mg/dL, ALT 205 U/L] and hemolytic anemia after 3 years of methyldopa therapy [Coombs positive, SMA positive]; delayed recovery on stopping methyldopa, but rapid response to prednisone and no recurrence upon withdrawal).
- Neuberger J, Kenna JG, Nouri Aria K, Williams R. Antibody mediated hepatocyte injury in methyl dopa induced hepatotoxicity. Gut. 1985;26:1233–9. [PMC free article: PMC1432918] [PubMed: 3905530](9 cases of methyldopa hepatotoxicity; 5 with acute liver failure, 3 acute self-limited hepatitis, 1 chronic active hepatitis arising 7 weeks to 3 years after starting methyldopa; 5 had antibody mediated cytotoxicity to rabbit hepatocytes exposed to methyldopa and a microsomal enzyme inducer).
- Otsuka M, Fujimura M, Koshino T, Ueda M, Otake S, Funada H, Harada M, et al. [A case of autoimmune hemolytic anemia, interstitial pneumonia and liver injury occurred during one month's medication of small dose of alpha-methyldopa] Rinsho Ketsueki 1985; 26: 1647-53. Japanese. PMID: 4094082. [PubMed: 4094082]
- Sakurai S, Yamada S, Nagamine T, Takezawa J, Ichikawa K, Arai T, Takagi H, et al. [A male case of methyldopa-induced liver injury with positive lupus cell preparation] Nippon Shokakibyo Gakkai Zasshi 1985; 82: 2134-8. Japanese. PMID: 2419608. [PubMed: 2419608]
- Minakami H, Ohkusa T, Kimura K, Tamada T, Hirota N. [Hepatic injury caused by methyldopa in a pre-eclamptic patient] Nippon Sanka Fujinka Gakkai Zasshi 1986; 38: 139-42. Japanese. PMID: 3950462. [PubMed: 3950462]
- Rao KV. Cholestatic jaundice associated with methyldopa. Minn Med. 1986;69:720–1. [PubMed: 3807865](40 year old man with probable alcoholic liver disease developed jaundice and cholestatic pattern of enzymes 2-3 months after restarting methyldopa [bilirubin 17.5 mg/dL, AST 60 U/L, Alk P 5 times ULN], resolving rapidly upon stopping).
- Stanley P, Mijch A. Methyldopa: an often overlooked cause of fever and transient hepatocellular dysfunction. Med J Aust. 1986;144:603–5. [PubMed: 3713591](Summary of 78 cases of methyldopa induced fever, onset in 5-35 days, no rash or eosinophilia, often have mild ALT elevations [~61%], occasionally hepatitis with jaundice [18%]).
- Olmos M, Guma C, Colombato LO, Lami G, Miyashiro R, Alvarez E. [Hepatic lesions induced by drugs. Report of 26 cases] Acta Gastroenterol Latinoam 1987; 17: 105-11. Spanish. PMID: 3442185. [PubMed: 3442185]
- Lee WM, Denton WT. Chronic hepatitis and indolent cirrhosis due to methyldopa: the bottom of the iceberg? J S C Med Assoc. 1989;85:75–9. [PubMed: 2918709](Among 15 cases of drug induced liver disease seen over a 2.5 year period, 6 were were due to methyldopa, including 2 with an acute [bilirubin 11-13 mg/dL, AST 500-1700 U/L, Alk P 247-345 U/L] and 4 a chronic presentation [bilirubin 0.3-3.1 mg/dL, AST 39-545 U/L, Alk P 121-280 U/L], all with SMA positivity, 3 presenting with cirrhosis [on methyldopa for 4-9 years]).
- Lee MG, Hanchard B, Williams NP. Drug-induced acute liver disease. Postgrad Med J. 1989;65:367–70. [PMC free article: PMC2429339] [PubMed: 2608576]
- Moses A, Zahger D, Amir G. Cholestatic liver injury after prolonged exposure to methyldopa. Digestion. 1989;42:57–60. [PubMed: 2744247](75 year old man developed jaundice having been on methyldopa for 6 years [bilirubin 26.3 mg/dL, ALT 960 U/L, Alk P 1120 U/L], resolving within 5 months of stopping).
- Picaud A, Walter P, de Préville G, Nicolas P. [Fatal toxic hepatitis in pregnancy. A discussion of the role of methyldopa] J Gynecol Obstet Biol Reprod (Paris) 1990; 19: 192-6. French. PMID: 2324442. [PubMed: 2324442]
- Mirada Canals A, Monteagudo Jimenez M, Sole Villa J, Rodriguez Moreno C. Methyldopa-induced granulomatous hepatitis. DICP. 1991;25:1269–70. [PubMed: 1763547]
- Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992;232:133–8. [PubMed: 1506809](Among 1100 liver adverse event reports in Denmark, methyldopa accounted for 13, ranking 17th).
- Daghfous R, el Aidli S, Loueslati MH, Sakka T, Takhal M, Ben Mami N, Ben Khelifa H, et al. [Methyldopa-induced hepatitis: 3 case reports] Tunis Med 1994; 72: 47-50. French. PMID: 8203031. [PubMed: 8203031]
- Smith GN, Piercy WN. Methyldopa hepatotoxicity in pregnancy: a case report. Am J Obstet Gynecol. 1995;172:222–4. [PubMed: 7847544](30 year old woman developed jaundice 3 weeks after starting methyldopa during pregnancy [bilirubin 6.6 mg/dL, ALT 2415 U/L], with slow recovery and then recurrence with reexposure).
- Pillans PI. Drug associated hepatic reactions in New Zealand: 21 years experience. N Z Med J. 1996;109:315–9. [PubMed: 8816722](Adverse event reporting over 20 year period from New Zealand identified 943 liver injuries from medications; top 10 drugs included methyldopa [n=38], which decreased in ranking from 3rd [before 1980] to 4th [1980-87] to <20th [1988-94]).
- Lammert F, Matern S. [Hepatic diseases caused by drugs] Schweiz Rundsch Med Prax 1997; 86: 1167-71. German. PMID: 9333916. [PubMed: 9333916]
- Thomas LA, Cardwell MS. Acute reactive hepatitis in pregnancy induced by alpha-methyldopa. Obstet Gynecol. 1997;90:658–9. [PubMed: 11770583](37 year old woman developed jaundice with hepatocellular pattern of enzymes 9 weeks after starting methyldopa therapy during pregnancy [bilirubin 13.9 mg/dL, ALT 898 U/L, Alk P 95 U/L], resolving within 4 weeks of stopping).
- Follmann M, Heinemann LA, Bauerfeind A, Garbe E. Treatment with potentially hepatotoxic drugs and the risk of hepatocellular carcinoma: results of a european case - control study. Pharmacoepidemiol Drug Saf. 2000;9:417–22. [PubMed: 19025848](Case control study of 317 cases of hepatocellular carcinoma found no association of cancer with taking medications including methyldopa).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, Garcia-Munoz B, et al. Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. [PubMed: 16083708](Reports to a Spanish network identified 570 cases of drug induced liver disease between 1994-2005, methyldopa not mentioned).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Survey of all cases of drug induced liver injury with fatal outcome from Swedish Adverse Drug Reporting system from 1966-2002; 103 cases identified, but only one was attributed to methyldopa).
- Fernández-Marcote Menor EM, Pérez-Bedmar Delgado J. [Methyldopa-induced acute toxic hepatitis] Rev Esp Enferm Dig 2005; 97: 840-1. Spanish. PMID: 16438629. [PubMed: 16438629](43 year old woman was given methyldopa during pregnancy and developed jaundice when she continued it afterwards [bilirubin 11 mg/dL, ALT 1904 U/L, ANA positive], resolving within 6 weeks of stopping).
- Phadnis SV, Sangay MR, Sanusi FA. Alpha-methyldopa-induced acute hepatitis in pregnancy. Aust N Z J Obstet Gynaecol. 2006;46:256–7. [PubMed: 16704485](40 year old woman developed fatigue within 2 weeks and jaundice within 4 weeks of starting methyldopa during pregnancy [bilirubin 2.0-5.4 mg/dL, ALT 2511 U/L, Alk P 216 U/L, ANA 1:180], resolving within 6 weeks of stopping).
- Podymow T, August P. Hypertension in pregnancy. Adv Chronic Kidney Dis. 2007;14:178–90. [PubMed: 17395120](Guidelines to therapy of hypertension during pregnancy).
- Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007;(1):CD002252. [PubMed: 17253478](Treatment of mild-to-moderate hypertension during pregnancy has little effect on outcomes; beta blockers are more effective than methyldopa in reducing blood pressure).
- Yusuff KB, Ajayi A, Joseph YB. Laboratory monitoring of hematological and hepatic parameters in ambulatory patients receiving alpha-methyldopa in a Nigerian tertiary care setting. Curr Drug Saf. 2008;3:163–6. [PubMed: 18690994](Retrospective chart review of whether ALT and AST monitoring was done in 260 patients given methyldopa; found no testing performed during first 6-12 weeks of therapy).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, 5 cases were attributed to methyldopa).
- Ali T, Srinivasan N, Le V, Rizvi S. Alpha-methyldopa hepatotoxicity in pregnancy. J Coll Physicians Surg Pak. 2009;19:125–6. [PubMed: 19208320](33 year old woman developed jaundice 6 weeks after starting methyldopa during pregnancy [bilirubin 19.9 mg/dL, ALT 1303 U/L, Alk P 134 U/L, ANA negative], resolving rapidly with prednisone treatment).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 2 due to methyldopa).
- Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M, Lindor K. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–8. [PubMed: 20512992](Retrospective analysis of 261 cases of autoimmune hepatitis, 24 [9%] of which were due to a medication; 11 nitrofurantoin and 11 minocylcine, but none due to methyldopa; drug induced cases resembled idiopathic cases in all regards except in ability to stop corticosteroids without relapse).
- Ozsvár Z, Solymossi Z, Monostory K. [Methyldopa-induced acute reactive hepatitis in pregnancy, drug-metabolizing capacity of the liver]. Orv Hetil 2010; 151: 457-61. Hungarian. PMID: 20211808. [PubMed: 20211808](A 35 year old pregnant woman developed hepatitis at gestational week 23 [bilirubin 6.1 mg/dL, ALT 1190 U/L, Alk P 266 U/L, ANA negative], resolving rapidly on stopping methyldopa therapy).
- Ozaslan E. Drug-induced autoimmune hepatitis: an easily reversible type of liver fibrosis? Hepatology. 2011;53:370. [PubMed: 20848612](Letter in response to Björnsson [2010] discussing the reversibility of early fibrosis in cases of drug induced autoimmune hepatitis).
- Slim R, Ben Salem C, Hmouda H, Bouraoui K. Hepatotoxicity of alpha-methyldopa in pregnancy. J Clin Pharm Ther. 2010;35:361–3. [PubMed: 20831537](A 34 year old woman developed jaundice and pruritus 4 weeks after starting methyldopa during pregnancy [bilirubin 9.4 mg/dL, ALT 685 U/L, Alk P 301 U/L], resolving within 10 weeks of stopping).
- Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011;56:958–76. [PubMed: 21327704](Review of drug induced autoimmune hepatitis, the principal causes being minocycline and nitrofurantoin; other causes were methyldopa, hydralazine, statins, fibrates, diclofenac, anti-TNF agents, interferons, propylthiouracil, and isoniazid).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period none of which were attributed to methyldopa or other antihypertensive medications).
- deLemos AS, Foureau DM, Jacobs C, Ahrens W, Russo MW, Bonkovsky HL. Drug-induced liver injury with autoimmune features. Semin Liver Dis. 2014;34:194–204. [PubMed: 24879983](Review of autoimmune drug induced liver injury which provides an example in a 39 year old African American woman treated with methyldopa shortly after pregnancy who developed jaundice 1 month later [bilirubin 19.9 mg/dL, ALT 1869 U/L, Alk P 205 U/L, ANA positive], resolving spontaneously within 6 months of stopping methyldopa).
- Kashkooli S, Baraty B, Kalantar J. α-Methyldopa-induced hepatitis during the postpartum period. BMJ Case Rep. 2014;2014:bcr2014203712. pii. [PMC free article: PMC3939412] [PubMed: 24577181](A 34 year old woman developed hepatitis two months after delivery and while on methyldopa [bilirubin 18.8 mg/dL, ALT 1018 U/L, Alk P 275 U/L, INR 1.2 rising to 1.7, ANA positive], resolving within 2 months of stopping drug).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, 2 were attributed to methyldopa, one of which was fatal).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. PMID: 25754159. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 39 [4%] were attributed to antihypertensive drugs including 11 to methyldopa).
- Koizumi T, Furuya K, Baba M, Sadaoka K, Sekiya C, Hattori A, Goto R, et al. [Case Report; A case of subacute fulminant hepatitis induced by methyldopa]. Nihon Naika Gakkai Zasshi 2015; 104: 586-9. Japanese. PMID: 26571747. [PubMed: 26571747](A 40 year old woman developed severe hepatitis while receiving methyldopa [bilirubin 18.4 mg/dL, ALT 1685 U/L, Alk P 833 U/L, INR 2.17, ANA positive], with progressive hepatic failure and death).
- Firoz T, Webber D, Rowe H. Drug-induced fulminant hepatic failure in pregnancy. Obstet Med. 2015;8:190–2. [PMC free article: PMC4935052] [PubMed: 27512479](A 39 year old pregnant woman developed jaundice 8 weeks after starting labetalol and 4 weeks after methyldopa [bilirubin 17.8 mg/dL, ALT 1406 U/L, Alk P 159 U/L, INR 3.1, ANA positive], resolving rapidly after stopping both medications and a complicated delivery).
- Stine JG, Northup PG. Autoimmune-like drug-induced liver injury: a review and update for the clinician. Expert Opin Drug Metab Toxicol. 2016;12:1291–301. [PubMed: 27402321](Review of drug induced liver injury with autoimmune features discusses methyldopa as a frequent cause).
- Chalasani N, Reddy KRK, Fontana RJ, Barnhart H, Gu J, Hayashi PH, Ahmad J, et al. Idiosyncratic drug induced liver injury in African-Americans is associated with greater morbidity and mortality compared to Caucasians. Am J Gastroenterol. 2017;112:1382–8. [PMC free article: PMC5667647] [PubMed: 28762375](Among 985 patients with drug-induced liver injury enrolled in a prospective US database between 2004 and 2016, methyldopa accounted for 4% of cases among 144 African Americans compared to <1% of 841 caucasians, and disease severity and worse outcomes were more frequent in African American subjects both overall and for methyldopa).
- de Boer YS, Kosinski AS, Urban TJ, Zhao Z, Long N, Chalasani N, Kleiner DE, Hoofnagle JH., Drug-Induced Liver Injury Network. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin Gastroenterol Hepatol. 2017;15:103–12.e2. [PMC free article: PMC5370577] [PubMed: 27311619](Analysis of 88 cases of liver injury due to medications known to induce autoimmune markers [including methyldopa, hydralazine, nitrofurantoin and minocycline] found that clinical features were similar in those with and those without an autoimmune phenotype and that HLA Class I and II alleles associated with spontaneous autoimmune hepatitis were not increased among patients with drug induced autoimmune liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Evidence that alpha-methylepinephrine is an antihypertensive metabolite of alpha-methyldopa.[Clin Exp Hypertens A. 1982]Evidence that alpha-methylepinephrine is an antihypertensive metabolite of alpha-methyldopa.Goldberg MR, Tung CS, Ring M, Oates JA, Gerkens JF, Robertson D. Clin Exp Hypertens A. 1982; 4(4-5):595-604.
- alpha-Methyldopa and depression: a clinical study and review of the literature.[Am J Psychiatry. 1983]alpha-Methyldopa and depression: a clinical study and review of the literature.DeMuth GW, Ackerman SH. Am J Psychiatry. 1983 May; 140(5):534-8.
- Absence of an effect of mianserin on the actions of clonidine or methyldopa in hypertensive patients.[Eur J Clin Pharmacol. 1983]Absence of an effect of mianserin on the actions of clonidine or methyldopa in hypertensive patients.Elliott HL, McLean K, Sumner DJ, Reid JL. Eur J Clin Pharmacol. 1983; 24(1):15-9.
- Review Methyldopa for primary hypertension.[Cochrane Database Syst Rev. 2009]Review Methyldopa for primary hypertension.Mah GT, Tejani AM, Musini VM. Cochrane Database Syst Rev. 2009 Oct 7; 2009(4):CD003893. Epub 2009 Oct 7.
- Review Clinical pharmacokinetics of methyldopa.[Clin Pharmacokinet. 1982]Review Clinical pharmacokinetics of methyldopa.Myhre E, Rugstad HE, Hansen T. Clin Pharmacokinet. 1982 May-Jun; 7(3):221-33.
- Methyldopa - LiverToxMethyldopa - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...