NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Methsuximide is a succinimide-based anticonvulsant similar to ethosuximide that is used for absence (petit mal) seizures in both adults and children. Methsuximide was associated with hepatic injury in experimental animals, but has not been linked to serum enzyme elevations during treatment or to cases of clinically apparent liver injury with jaundice.
Background
Methsuximide (meth sux’ i mide: also called mesuximide) is a succinimide derivative and anticonvulsant that is used to treat absence (petit mal) seizures. The succinimide anticonvulsants reduce low threshold calcium currents in thalamic neurons, and suppress the paroxysmal spike and wave activity that is associated with lapses of consciousness that occur with absence seizures. Methsuximide was first approved in 1957 for use alone or in combination with other agents to treat absence seizures. Methsuximide is no longer in common use, having been replaced by ethosuximide, the most active of the succinimide anticonvulsants and first line therapy for petit mal epilepsy. Nevertheless, methsuximide is still available, generically and under the brand name Celontin as capsules of 150 and 300 mg. The recommended initial dose in adults and children above the age of 6 years is 300 mg once daily, with gradual dose escalation weekly based upon tolerance and effect to a maximum of 1200 mg daily. Common side effects include dizziness, somnolence, ataxia, fatigue, irritability, anorexia and epigastric discomfort.
Hepatotoxicity
Prospective studies suggest that chronic methsuximide therapy is not accompanied by significant elevations in serum aminotransferase levels, although it has been shown to have hepatotoxicity in animals. Clinically apparent hepatotoxicity from methsuximide has not been reported, although hypersensitivity reactions with fever and rash are not uncommon (1% to 5%). The product label for methsuximide warns of hepatic dysfunction and recommends periodic liver function studies. Nevertheless, clinically apparent liver injury from methsuximide must be very rare, if it occurs at all.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
Methsuximide is metabolized to inactive intermediates in the liver via the cytochrome P450 system (CYP 3A4) and is susceptible to many drug-drug interactions.
Outcome and Management
While there have been no reported instances of liver injury from methsuximide, monitoring for liver tests is recommended. While there may be cross reactivity to hypersensitivity reactions or liver injury between methsuximide and ethosuximide, there is no evidence of cross reactivity with the aromatic anticonvulsants.
Drug Class: Anticonvulsants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Methsuximide – Generic, Celontin®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
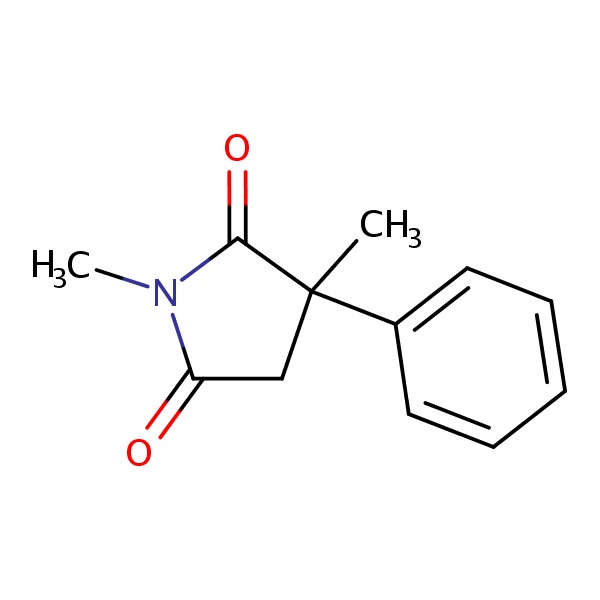
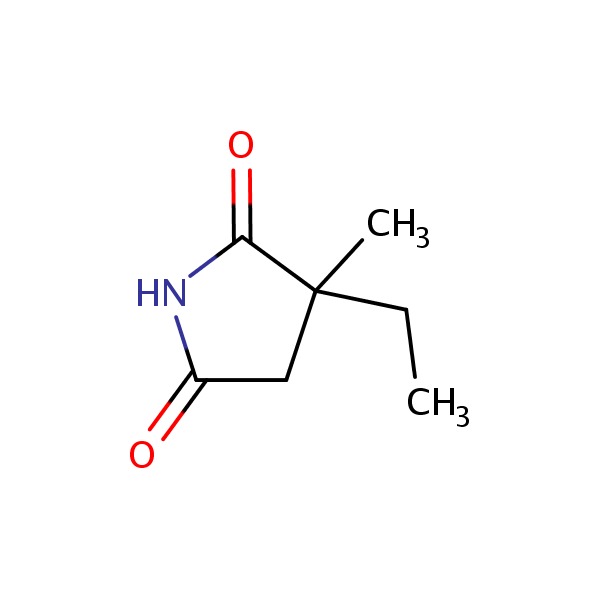
CHEMICAL FORMULAS AND STRUCTURES
ANNOTATED BIBLIOGRAPHY
References updated: 01 July 2016
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999; methsuximide is not discussed).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-42.(Review of anticonvulsant induced liver injury; methsuximide is not discussed).
- McNamara JO. Pharmacology of the epilepsies. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 583-607.(Textbook of pharmacology and therapeutics).
- Livingston S, Pauli L. Celontin (PM-396) in the treatment of epilepsy. Pediatrics 1957; 19 (4 Pt 1): 614-8. [PubMed: 13419432](Among 136 patients with various types of poorly controlled seizures treated with methsuximide for 6-15 months, beneficial results were found largely in those with minor motor and psychomotor seizures, and routine blood tests were reported to remain normal).
- French EG, Rey-Bellet J, Lennox WG. Methsuximide in psychomotor and petit-mal seizures. N Engl J Med 1958; 258: 892-4. [PubMed: 13541681](Among 106 adults and children with varying types of poorly controlled seizures treated with methsuximide, 30 dropped out and 49 [48%] reported some degree of improvement, side effects being drowsiness, nausea and vomiting, constipation, rash [n=9], confusion and headaches; no mention of jaundice or hepatotoxicity).
- Karch SB. Methsuximide overdose. Delayed onset of profound coma. JAMA 1973; 223: 1463-5. [PubMed: 4740029](18 year old girl with epilepsy took an overdose of 10 g of methsuximide and developed dizziness, followed by somnolence and then deep coma 9 hours later, persisting for 72 hours; ALT, AST, Alk P and bilirubin levels were normal).
- Reynolds NC Jr, Miska RM. Safety of anticonvulsants in hepatic porphyrias. Neurology 1981; 31: 480-4. [PubMed: 7194443](Mentions that both methsuximide and ethosuximide have been associated with triggering attacks of acute intermittent porphyria).
- Wilder BJ, Buchanan RA. Methsuximide for refractory complex partial seizures. Neurology 1981; 31: 741-4. [PubMed: 6894634](Among 21 patients with refractory complex partial seizures treated with methsuximide, 8 had a complete and 7 a partial response; side effects included somnolence, headache, ataxia, skin rash, nausea and malaise, but “most were not serious”; no mention of hepatotoxicity or serum enzyme elevations).
- Coulter DL. Ethosuximide-induced liver dysfunction. Arch Neurol 1983; 40: 393-4. [PubMed: 6847455](1 year old boy developed ALT elevations one month after ethosuximide was added to a chronic regimen of valproate and phenobarbital that continued to worsen after stopping valproate [bilirubin normal, ALT 69 rising 229 U/L, GGT 175 U/], resolving rapidly when ethosuximide was stopped).
- Browne TR, Feldman RG, Buchanan RA, Allen NC, Fawcett-Vickers L, Szabo GK, Mattson GF, et al. Methsuximide for complex partial seizures: efficacy, toxicity, clinical pharmacology, and drug interactions. Neurology 1983; 33: 414-8. [PubMed: 6403891](Among 26 adults with poorly controlled, complex partial seizures treated with methsuximide, 8 had a clinical response and continued on therapy; side effects included drowsiness, gastrointestinal complaints, hiccups, irritability, headache and ataxia, but there was “no serious idiosyncratic hemopoietic, hepatic, or renal toxicity”).
- Tennison MB, Greenwood RS, Miles MV. Methsuximide for intractable childhood seizures. Pediatrics 1991; 87: 186-9. [PubMed: 1987529](Among 23 children with various forms of intractable seizures treated with methsuximide, 15 had a 50% or greater decrease in seizures; 4 had side effects requiring discontinuation, include 2 with rash; no details provided).
- Hurst DL. Methsuximide therapy of juvenile myoclonic epilepsy. Seizure 1996; 5: 47-50. [PubMed: 8777552](Among 5 adolescent girls with juvenile myoclonic epilepsy treated with methsuximide, all became seizure free and 4 could stop all other anticonvulsants; there were no serious adverse events).
- Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug Saf 1999; 21: 489– 501. [PubMed: 10612272](Review of anticonvulsant hypersensitivity syndrome: triad of fever, rash and internal organ injury occurring 1-8 weeks after exposure to anticonvulsant, liver being most common internal organ involved. Occurs in 1:1000-1:10,000 initial exposures to phenytoin, carbamazepine, phenobarbital or lamotrigine, unrelated to dose, perhaps predisposed by valproate; liver injury arises 1-4 weeks after onset of rash and ranges in severity from asymptomatic ALT elevations to icteric hepatitis to ALF; high mortality rate with jaundice; other organs include muscle, kidney, brain, heart and lung. Pseudolymphoma syndrome and serum sickness like syndrome are separate complications of anticonvulsants; role of corticosteroids uncertain; cross reactivity among the agents should be assumed; methsuximide is not mentioned).
- Hamer HM, Morris HH. Hypersensitivity syndrome to antiepileptic drugs: a review including new anticonvulsants. Clevel Clin J Med 1999; 66: 239-45. [PubMed: 10199060](Clinical review of anticonvulsant syndrome, which occurs in 1-5/10,000 users, higher risk in African Americans and affected siblings; liver involvement common, but most cases are anicteric; other manifestations include facial edema, lymphadenopathy, bone marrow aplasia, pseudolymphoma, thyroiditis, interstitial nephritis, switching to valproate, levetiracetam or benzodiazepines is safe; methsuximide is not mentioned).
- Sigler M, Strassburg HM, Boenigk HE. Effective and safe but forgotten: methsuximide in intractable epilepsies in childhood. Seizure 2001; 10: 120-4. [PubMed: 11407955](Among 112 children with intractable epilepsy treated with methsuximide, there were no serious or irreversible adverse events and 50% had a decrease in seizure frequency).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 10 were attributed to phenytoin, 10 to valproate and 1 to carbamazepine, but none to ethosuximide or methsuximide).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scan 2008; 118: 281-90. [PubMed: 18341684](Review of all anticonvulsants; mentions that there is no known clinically significant hepatotoxicity associated with ethosuximide).
- Penovich PE, Willmore LJ. Use of a new antiepileptic drug or an old one as first drug for treatment of absence epilepsy. Epilepsia 2009; 50 Suppl 8: 37-41. [PubMed: 19702732](Review of the therapy of absence seizures, mentions that side effects of ethosuximide "tend to be a challenge"; no mention of methsuximide or of hepatotoxicity).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 [11%] were attributed to drug induced liver injury of which 11 [8%] were due to anticonvulsants, including phenytoin [8], valproate [2], and carbamazepine [3], but none were attributed to ethosuximide or methsuximide).
- Hoepner R, May TW, Rambeck B, Ottenottebrock H, Valentin R, Brandt C. Symptoms and course of intoxication with mesuximide--a case report. Epilepsy Behav 2012; 25: 129-30. [PubMed: 22818365](31 year old woman on multiple antiepileptic drugs developed worsening somnolence and was found to have abnormally elevated methsuximide levels; no mention of ALT elevations or hepatotoxicity).
- Knowles SR, Dewhurst N, Shear NH. Anticonvulsant hypersensitivity syndrome: an update. Expert Opin Drug Saf 2012; 11: 767-78. [PubMed: 22794330](Updated review of anticonvulsant hypersensitivity syndrome associated with phenytoin, phenobarbital, lamotrigine and carbamazepine and rarely with zonisamide, valproate and oxcarbazepine; methsuximide is not mentioned).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury from the US enrolled in a prospective database between 2004 and 2012, anticonvulsants were implicated in 40 cases, including one attributed to ethosuximide, but none to methsuximide).
- Drugs for epilepsy. Treat Guidel Med Lett 2013; 11: 9-18. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants; mentions that ethosuximide is the first line drug recommended for treatment of absence seizures, but does not mention methsuximide).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Ethosuximide.[LiverTox: Clinical and Researc...]Review Ethosuximide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Gas-chromatographic analysis for succinimide anticonvulsants in serum: macro- and micro-scale methods.[Clin Chem. 1976]Gas-chromatographic analysis for succinimide anticonvulsants in serum: macro- and micro-scale methods.Bonitati J. Clin Chem. 1976 Mar; 22(3):341-5.
- MECHANISM OF ANTICONVULSANT ACTION OF KETOGENIC DIET. STUDIES IN ANIMALS WITH EXPERIMENTAL SEIZURES AND IN CHILDREN WITH PETIT MAL EPILEPSY.[Am J Dis Child. 1964]MECHANISM OF ANTICONVULSANT ACTION OF KETOGENIC DIET. STUDIES IN ANIMALS WITH EXPERIMENTAL SEIZURES AND IN CHILDREN WITH PETIT MAL EPILEPSY.MILLICHAP JG, JONES JD, RUDIS BP. Am J Dis Child. 1964 Jun; 107:593-604.
- [ETHOSUXIMIDE (ZARONDAN) IN THE TREATMENT OF PETIT MAL EPILEPSY AND RELATED SEIZURES].[Ugeskr Laeger. 1964][ETHOSUXIMIDE (ZARONDAN) IN THE TREATMENT OF PETIT MAL EPILEPSY AND RELATED SEIZURES].PALUDAN J, KIORBOE E, TROLLE E, OVERVAD E. Ugeskr Laeger. 1964 Feb 13; 126:201-3.
- Review Genetic animal models of epilepsy as a unique resource for the evaluation of anticonvulsant drugs. A review.[Methods Find Exp Clin Pharmaco...]Review Genetic animal models of epilepsy as a unique resource for the evaluation of anticonvulsant drugs. A review.Löscher W. Methods Find Exp Clin Pharmacol. 1984 Sep; 6(9):531-47.
- Methsuximide - LiverToxMethsuximide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...