NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Mepenzolate is an anticholinergic agent used to treat gastrointestinal conditions such as acid peptic disease and irritable bowel syndrome. Mepenzolate has not been implicated in causing liver enzyme elevations or clinically apparent acute liver injury.
Background
Mepenzolate (me pen' zoe late) is a synthetic quaternary ammonium anticholinergic agent which inhibits the muscarinic actions of acetylcholine on autonomic nerve endings, decreasing gastrointestinal secretions and intestinal motility. Mepenzolate has broad activity against muscarinic acetylcholine receptors, but its highly polar quaternary ammonium group makes it less likely to cross lipid membranes such as the blood brain barrier, which is believed to decrease the potential for central nervous system effects. Mepenzolate has been used largely for gastrointestinal conditions including peptic ulcer disease and gastrointestinal conditions associated with pain and spasm. Mepenzolate is approved for use in the United States for the treatment of peptic ulcer disease but is now not commonly used, having been replaced by more effective antiulcer agents. Mepenzolate is available in tablets 25 mg under the brand name Cantil. The typically recommended oral dose in adults is 25 to 50 mg two to four times daily. Common side effects are those of parasympathetic stimulation and include dryness of the mouth and eyes, decreased sweating, headache, visual blurring, constipation, and urinary retention. Because of its structure, mepenzolate is believed to be less likely than other anticholinergics to cross the blood brain barrier and cause central nervous system effects such as restlessness, confusion and hallucinations. Anticholinergic agents can precipitate acute narrow angle glaucoma and acute urinary retention.
Hepatotoxicity
Like other anticholinergic agents, mepenzolate has not been linked to episodes of liver enzyme elevations or clinically apparent liver injury. The metabolism of mepenzolate is not well defined, but it is likely metabolized by the liver.
References on the safety and potential hepatotoxicity of anticholinergics are given together after the Overview section on Anticholinergic Agents.
Drug Class: Anticholinergic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Mepenzolate – Cantil®
DRUG CLASS
Anticholinergic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Mepenzolate | 25990-43-6 | C21-H26-N-O3 |
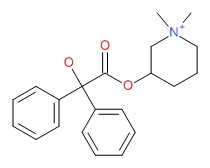 |
- Review Dicyclomine.[LiverTox: Clinical and Researc...]Review Dicyclomine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Ameliorative effect of mepenzolate bromide against pulmonary fibrosis.[J Pharmacol Exp Ther. 2014]Ameliorative effect of mepenzolate bromide against pulmonary fibrosis.Kurotsu S, Tanaka K, Niino T, Asano T, Sugizaki T, Azuma A, Suzuki H, Mizushima T. J Pharmacol Exp Ther. 2014 Jul; 350(1):79-88. Epub 2014 Apr 25.
- Review Glycopyrrolate.[LiverTox: Clinical and Researc...]Review Glycopyrrolate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Propantheline.[LiverTox: Clinical and Researc...]Review Propantheline.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Superiority of pulmonary administration of mepenzolate bromide over other routes as treatment for chronic obstructive pulmonary disease.[Sci Rep. 2014]Superiority of pulmonary administration of mepenzolate bromide over other routes as treatment for chronic obstructive pulmonary disease.Tanaka K, Kurotsu S, Asano T, Yamakawa N, Kobayashi D, Yamashita Y, Yamazaki H, Ishihara T, Watanabe H, Maruyama T, et al. Sci Rep. 2014 Mar 28; 4:4510. Epub 2014 Mar 28.
- Mepenzolate - LiverToxMepenzolate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...