NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Marijuana is an extract from the twigs, seeds and fruit of the Cannabis sativa plant which is widely used for its psychoactive effects and is illegal in many areas of the world including at least half of the United States. Recently, marijuana has been purported to have beneficial medicinal effects including antiemetic, analgesic, anxiolytic, sedative, sleep inducing, and antidepressant activities. Marijuana use has not been linked to serum enzyme elevations during therapy or to instances of clinically apparent liver injury with jaundice.
Background
Marijuana (mare” a wan’ a) is a psychoactive extract of the small twigs, seeds and fruit of female marijuana plants (Cannabis sativa), a large, hardy, annual or biennial plant that originated from the Middle East but is now found worldwide in temperate and tropical areas. While marijuana is often referred to as cannabis, the term cannabis actually refers to all Cannabis sativa products, both psycho-active and -neutral. More than 50 cannabinoids have been identified in Cannabis sativa extracts, but the most abundant is delta-9 tetrahydrocannabinol (THC) which appears to be the major psychoactive component of marijuana. THC binds to the cannabinoid receptors that are found in the central nervous system (CB1 receptors), but also the periphery (largely CB2 receptors). Activation of CB receptors results in potent effects on appetite, mood, cognition, memory and perception and possibly on pain, inflammatory, and metabolic pathways. Purified or synthetic THC are the main components of dronabinol and nabilone, FDA approved prescription drugs that are used for the treatment of the nausea and vomiting associated with anticancer therapy and for anorexia and weight loss in patients with AIDS. Cannabis and THC preparations have multiple physiological effects besides antiemetic and appetite stimulating actions, including sedation, reduction of anxiety and restlessness, and decrease in intraocular pressure. Less well established effects include use as a muscle relaxant or analgesic and as treatment for depression, epilepsy and Tourette syndrome. Cannabis also causes euphoria and altered mentation and is widely used recreationally for these effects. Marijuana and most cannabinoids are poorly absorbed by mouth and subject to first pass uptake by the liver, for which reason it is typically smoked or vaped which gives it maximal systemic exposure. Sale of marijuana or THC is banned in many countries including by the US Federal Government. Nevertheless, it is legalized for production and sale within several states. Recently, medical marijuana has been approved in some countries and parts of the United States generally for conditions such as chronic pain, nausea and vomiting, arthritis, anxiety and depression. Similarly, recreational marijuana has now been decriminalized and even legalized in many countries and States of the Union. However, US Federal laws still label cannabis as an illegal drug, outlaw its interstate commerce, and not proven to have medical effectiveness for any disease or condition. Marijuana is available in multiple forms, including extracts or solutions for smoking and vaping, in foods and gummies, and as powders, dry extracts, tablets or capsules. These forms of cannabis are available over-the-counter or as illegal products but are generally of uncertain purity, potency and safety. Common side effects include fatigue, sedation, somnolence, dizziness, euphoria, abnormal thinking, paranoid reactions, impairment of driving and operation of heavy equipment, conjunctivitis, diarrhea, nausea, vomiting, abdominal pain, orthostatic hypotension and tachycardia. Rare side effects include hallucinations and seizures. Marijuana, THC and cannabis have a clear potential for physical and psychological dependency and abuse, sometimes referred to as cannabis use disorder.
Hepatotoxicity
The frequency and severity of serum enzyme and bilirubin elevations during cannabis use are not well defined, but in small prospective studies, no biochemical abnormalities were found with its use. Furthermore, while rare case reports of acute liver injury attributed to marijuana have been reported, none were convincing or well documented. In large case series of drug and herbal supplement induced liver injury, cannabis and marijuana have not been implicated. In large epidemiologic studies of populations, cannabis use has been repeatedly linked to liver abnormalities, but all such studies were retrospective and uncontrolled, particularly for the presence of other causes of chronic liver injury. Careful, prospective controlled studies are needed to resolve the issue, but at present cannabis does not appear to cause acute liver injury or to exacerbate preexisting liver disease.
Likelihood score: E (Unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Cannabis is metabolized by the liver by the microsomal cytochrome P450 system, predominant CYP 2D6 and 3A4. It is not well absorbed orally and undergoes extensive first-pass metabolism to both active and inactive metabolites. Despite its hepatic metabolism, it has not been implicated in causing clinically significant drug-drug interactions. The lack of reported convincing cases of liver injury and low rate of drug-drug interactions due to cannabis may be due to the low doses and limited duration of typical therapy.
Other names: Grass, Indian Hemp, Pot, Weed, Mary Jane
Drug Class: Sedatives and Hypnotics, Antiemetic Agents
Other Related Cannabinoid Agents: Dronabinol, Nabilone
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Marijuana – Generic
DRUG CLASS
Antiemetic Agents
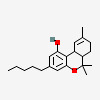
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Δ9Tetrahydrocannabinol | 16849-50-6 | C21-H30-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 16 February 2023
Abbreviations used: C AIDS, acquired immune deficiency syndrome; B, cannabinoids; CBD, cannabidiol; THC, Δ9 tetrahydrocannabinol.
- Zimmerman HJ. Antiemetic and prokinetic compounds. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 721.(Expert review of hepatotoxicity published in 1999 does not discuss cannabis or marijuana).
- Sharkey KA, MacNaughton WK. Antinauseants and antiemetics. Gastrointestinal motility bowel motility and water flux, emesis, and biliary and pancreatic disease. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 934-8.(Textbook of pharmacology and therapeutics).
- Marijuana. In, PDR for Herbal Medicines. 4th ed. Montvale, New Jersey: Thomson Healthcare Inc. 2007: pp. 562-7.(Compilation of short monographs on herbal medications and dietary supplements, in the discussion of adverse events associated with cannabis there is no mention of hepatotoxicity).
- NCCIH. https://www
.nccih.nih .gov/health/cannabis-marijuana-and-cannabinoids-what-you-need-to-know. (Website of the National Center for Complementary and Integrated Health [NCCIH], which includes factsheets on many herbal products including marijuana, with descriptions of its potential clinical effects and risks of side effects). - Kew MC, Bersohn I, Siew S. Possible hepatotoxicity of cannabis. Lancet. 1969;1(7594):578–9. [PubMed: 4179872](Letter to the editor describing a 21 year old man with cirrhosis of unknown cause who was a frequent user of marijuana; testing of 12 frequent marijuana users revealed mild, nonspecific elevations in liver related tests in 8 which were not explained by other diagnoses).
- Borini P, Guimarães RC, Borini SB. Possible hepatotoxicity of chronic marijuana usage. Sao Paulo Med J. 2004;122:110–6. [PubMed: 15448809](Among 123 marijuana users, liver test abnormalities were frequent, including serum ALT elevations in 34% of those who used marijuana only and 71% of those who also drank alcohol; the liver abnormalities were mostly mild and without jaundice or symptoms; no mention of tests for hepatitis B or C).
- Hézode C, Roudot-Thoraval F, Nguyen S, Grenard P, Julien B, Zafrani ES, Pawlotsky JM, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42:63–71. [PubMed: 15892090](Among 270 patients with chronic hepatitis C undergoing liver biopsy and evaluation for marijuana use and alcohol intake, daily cannabis use was associated with more advanced fibrosis; other risk factors were disease activity, duration of infection, genotype 3, steatosis, and alcohol intake).
- Hézode C, Zafrani ES, Roudot-Thoraval F, Costentin C, Hessami A, Bouvier-Alias M, Medkour F, et al. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology. 2008;134:432–9. [PubMed: 18242211](Among 315 patients with chronic hepatitis C undergoing liver biopsy and evaluation for marijuana use and alcohol intake, daily cannabis smoking was independently associated with hepatic steatosis even after controlling for alcohol intake and genotype 3 infection).
- Ishida JH, Peters MG, Jin C, Louie K, Tan V, Bacchetti P, Terrault NA. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol. 2008;6:69–75. [PMC free article: PMC3184401] [PubMed: 18166478](Among 204 patient with chronic hepatitis C undergoing liver biopsy, daily cannabis use was associated with more advanced fibrosis in multivariant analysis).
- Le Strat Y, Le Foll B. Obesity and cannabis use: results from 2 representative national surveys. Am J Epidemiol. 2011;174:929–33. [PubMed: 21868374](Analysis of two national surveys demonstrated a lower prevalence of obesity among frequent marijuana users than in non-users [14.3% vs 22% in survey 1 and 27.2% vs 25.3% in survey 2]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, no cases were attributed to marijuana or other cannabinoids).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none of these were attributed to marijuana or other cannabinoids).
- Swarnalatha G, Pai S, Ram R, Dakshinamurty KV. Fulminant hepatic failure following marijuana drug abuse: Molecular adsorbent recirculation system therapy. Indian J Nephrol. 2013;23:384–6. [PMC free article: PMC3764719] [PubMed: 24049281](23 year old male developed fatigue followed by acute liver failure [bilirubin 13 mg/dL, ALT 1346 U/L, Alk P 508 U/L, protime time 85 seconds] with recovery after treatment with a molecular adsorbent recirculation system [MARS], liver injury being attributed to marijuana because of a positive urine test for THC and absence of evidence for another cause).
- Borgelt LM, Franson KL, Nussbaum AM, Wang GS. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33:195–209. [PubMed: 23386598](Review of history and status of cannabis used for medical purposes including severe pain, muscle spasms, anorexia, nausea and vomiting, and glaucoma; adverse events were common, but usually mild and reversible, most frequently dry mouth, dizziness, drowsiness, and changes in cognition and mood).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to cannabis or other cannabinoid).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common implicated agents being nimesulide [n=53: 30%], cyproterone [n=18], nitrofurantoin [n=17], antituberculosis drugs [n=13], and flutamide [n=12: 7%]; no cannabinoid was listed).
- Sheikh IA, Lukšič M, Ferstenberg R, Culpepper-Morgan JA. Spice/K2 synthetic marijuana-induced toxic hepatitis treated with N-acetylcysteine. Am J Case Rep. 2014;15:584–8. [PMC free article: PMC4282190] [PubMed: 25548903](45 year old man developed acute hepatitis a week after starting the synthetic recreational marijuana “Spice/K2” [initial bilirubin 0.7 rising to 7.0 mg/dL, ALT 799 rising to 4136U/L, Alk P 260 U/L], with recovery over the next two weeks).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to cannabis or other cannabinoids).
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–73. [PubMed: 26103030](A systematic review of 79 studies of the medical use of cannabinoids for various conditions, found evidence of benefit in most trials, but differences from placebo often did not reach statistical significance; rates of response in trials for nausea and vomiting were 47% vs 20% and for pain were 37% vs 31%, while adverse events were generally more frequent with cannabinoids, most frequently dizziness, dry mouth, fatigue, nausea, somnolence, euphoria, disorientation, drowsiness, confusion, ataxia and hallucinations; no mention of ALT elevations or hepatotoxicity).
- Adejumo AC, Alliu S, Ajayi TO, Adejumo KL, Adegbala OM, Onyeakusi NE, Akinjero AM, et al. Cannabis use is associated with reduced prevalence of non-alcoholic fatty liver disease: A cross-sectional study. PLoS One. 2017;12:e0176416. [PMC free article: PMC5404771] [PubMed: 28441459](A population based case-control study of patients using the Healthcare Cost and Utilization Project Nationwide Inpatient Sample demonstrated a lower rate of nonalcoholic fatty liver disease in cannabis users, particularly cannabis dependent patients suggesting that its use might lessen fatty liver disease injury).
- Adejumo AC, Adegbala OM, Adejumo KL, Bukong TN. Reduced incidence and better liver disease outcomes among chronic HCV infected patients who consume cannabis. Can J Gastroenterol Hepatol. 2018;2018:9430953. [PMC free article: PMC6174743] [PubMed: 30345261](A case-control study of patients using the Healthcare Cost and Utilization Project Nationwide Inpatient Sample between 2007 and 2014 demonstrated a lower rate of cirrhosis among hepatitis C virus infected individuals who were cannabis users, although rates of hepatocellular carcinoma and HCV related deaths were similar in users as non-users).
- Adejumo AC, Ajayi TO, Adegbala OM, Adejumo KL, Alliu S, Akinjero AM. Cannabis use is associated with reduced prevalence of progressive stages of alcoholic liver disease. Liver Int. 2018;38:1475–1486. Onyeakus i NE, et al. [PubMed: 29341392](A population based case-control study of patients using the 2014 Healthcare Cost and Utilization Project Nationwide Inpatient Sample demonstrated lower rate of alcoholic steatosis, steatohepatitis, cirrhosis and hepatocellular carcinoma among patients with alcohol use disorder who were also cannabis users, although the effect was not seen in patients with the highest daily alcohol intake).
- Polito S, MacDonald T, Romanick M, Jupp J, Wiernikowski J, Vennettilli A, Khanna M, et al. Safety and efficacy of nabilone for acute chemotherapy-induced vomiting prophylaxis in pediatric patients: A multicenter, retrospective review. Pediatr Blood Cancer. 2018;65:e27374. [PubMed: 30051617](Among 110 pediatric patients treated with nabilone for prevention of chemotherapy induced nausea and vomiting [usually in combination with a serotonin [5-HT3] receptor antagonist], adverse events arose in 34%, most commonly sedation [20%], dizziness [10%] and euphoria [4%], leading to discontinuation in 9%, but without serious adverse events; no mention of ALT elevations or hepatotoxicity).
- Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159:1932–1954. [PubMed: 29847469](Systematic review of 91 publications including 104 studies and 9958 participants in trials of cannabinoids for pain reduction reported significant pain reduction in 29% of cannabinoid recipients vs 26% of placebo recipients, although adverse event rates were higher with cannabis products; no mention of specific adverse events, ALT elevations or hepatotoxicity).
- Torres-Moreno MC, Papaseit E, Torrens M, Farré M. Assessment of efficacy and tolerability of medicinal cannabinoids in patients with multiple sclerosis: a systematic review and meta-analysis. JAMA Netw Open. 2018;1:e183485. [PMC free article: PMC6324456] [PubMed: 30646241](A systematic review of 17 randomized controlled trials of various cannabinoids in multiple sclerosis found limited therapeutic efficacy for spasticity, pain and bladder dysfunction and higher rates of adverse events including dizziness, dry mouth, fatigue, ataxia, impaired memory, and somnolence, but similar rates of severe adverse events; no mention of ALT elevations or hepatotoxicity).
- Herrmann N, Ruthirakuhan M, Gallagher D, Verhoeff NPLG, Kiss A, Black SE, Lanctôt KL. Randomized placebo-controlled trial of nabilone for agitation in Alzheimer's Disease. Am J Geriatr Psychiatry. 2019;27:1161–1173. [PubMed: 31182351](Among 38 elderly patients with Alzheimer disease treated with nabilone [titrated] or placebo for 6 weeks in a cross-over trial, nabilone therapy was associated with a slight decrease in agitation but increase in sedation; no mention of ALT elevations or hepatoxicity).
- Cannabis and cannabinoids. Med Lett Drugs Ther. 2019;61(1585):179–182. [PubMed: 31770357](Concise review of the beneficial and adverse effects of cannabis and cannabinoids, 3 of which are FDA approved products, two synthetic agents that are used for prevention of chemotherapy induced nausea and vomiting [nabilone and dronabinol] and one a purified natural product used to treat rare forms of severe epilepsy [cannabidiol]; cannabis can cause dizziness, somnolence, euphoria, abnormal thinking, difficulty concentrating, hypotension, hypertension, syncope and tachycardia as well as addiction and dependence with long term use; no mention of ALT elevations or hepatoxicity).
- Bar-Lev Schleider L, Mechoulam R, Saban N, Meiri G, Novack V. Real life experience of medical cannabis treatment in autism: analysis of safety and efficacy. Sci Rep. 2019;9:200. [PMC free article: PMC6336869] [PubMed: 30655581](Among 188 patients with autism spectrum disorders treated with miscellaneous cannabis products [usually consisting of 30% CBD and 1.5% THC, given 3 times daily] between 2015 and 2017 in Israel, 49% reported significant improvement, 31% moderate improvement, 6% side effects and 14% no change; no mention of ALT elevations or hepatotoxicity).
- Drugs for anxiety disorders. Med Lett Drugs Ther. 2019;61(1578):121–126. [PubMed: 31386647](Concise review of drugs used to treat anxiety including cannabidiol products which are marketed as effective but “their potency, purity, efficacy and safety are unknown”).
- Chesney E, McGuire P, Freeman TP, Strang J, Englund A. Lack of evidence for the effectiveness or safety of over-the-counter cannabidiol products. Ther Adv Psychopharmacol. 2020;10:2045125320954992. [PMC free article: PMC7491225] [PubMed: 32973998](Extensive review of the efficacy and safety of cannabidiol compares pharmaceutical grade to over-the-counter grade products, most of which are of uncertain purity and concentration and are used in lower doses [<300 mg/day], which are unlikely to achieve plasma levels associated with engagement of cannabinoid receptors and which have not consistently shown the effects of the higher doses of pharmaceutical grade cannabidiol that has been shown to reduce anxiety and psychiatric symptoms but also cause liver enzyme elevations, particularly when given with clobazam or valproate).
- Sholler DJ, Huestis MA, Amendolara B, Vandrey R, Cooper ZD. Therapeutic potential and safety considerations for the clinical use of synthetic cannabinoids. Pharmacol Biochem Behav. 2020;199:173059. [PMC free article: PMC7725960] [PubMed: 33086126](Extensive review of the endocannabinoid system and potential therapies for synthetic cannabinoids [CB] and CB receptor antagonists; while there are CB receptors in the liver [predominantly CB2], there is no evidence that the CBs in clinical practice cause liver injury).
- Grimison P, Mersiades A, Kirby A, Lintzeris N, Morton R, Haber P, Olver I, et al. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: a randomised, placebo-controlled, phase II crossover trial. Ann Oncol. 2020;31:1553–1560. [PubMed: 32801017](Among 81 patients with refractory chemotherapy induced nausea and vomiting were treated with 1 to 4 oral cannabis extract tablets [THC 2.5 mg/CBD 2.5 mg] or placebo three times daily 1 day before and 5 days after chemotherapy in a crossover design, a complete response [no nausea or vomiting] occurred in 25% of THC/CBD treated compared to 14% of placebo treated courses; adverse events more frequent with THC/CBD included sedation, dizziness, and disorientation, but there were no cannabinoid related serious adverse events and no mention of ALT elevations or hepatotoxicity).
- Bouquet E, Pain S, Eiden C, Jouanjus E, Richard N, Fauconneau B, Pérault-Pochat MC., French Addictovigilance Network. Adverse events of recreational cannabis use reported to the French Addictovigilance Network (2012-2017). Br J Clin Pharmacol. 2021;87:3925–3937. [PubMed: 34282851](Among 2217 patients enrolled in a French vigilance registry of addiction involving cannabis use between 2012 and 2017, most were men [76%], and 18 to 34 years old [57%], the most frequent adverse events were psychiatric [51%], neurological [37%], cardiac [8%], and cannabinoid hyperemesis syndrome [3%], with only 3 being hepatobiliary events [abscess, sclerosing cholangitis and “cytolysis”], with no unexpected hepatic events, or deaths from liver disease).
- Watkins PB, Church RJ, Li J, Knappertz V. Cannabidiol and abnormal liver chemistries in healthy adults: results of a phase I clinical trial. Clin Pharmacol Ther. 2021;109:1224–1231. [PMC free article: PMC8246741] [PubMed: 33022751](Among 16 healthy volunteers treated with cannabidiol in doses of 1500 mg daily for 3 to 4 weeks, 7 [44%] developed ALT elevations which were greater than 5 times ULN in 5 [31%] beginning with 2-4 weeks and resolving rapidly with discontinuation; no mention of bilirubin elevations but alkaline phosphatase elevations occurred as well and some patients developed symptoms or signs of hepatitis including fever, nausea, abdominal discomfort, and eosinophilia).
- Yan K, Forman L. Cannabinoid use among liver transplant recipients. Liver Transpl. 2021;27:1623–1632. [PubMed: 34018308](Among 1227 liver transplant recipients, 538 responded to an exploratory survey on cannabinoid use, 24% were using marijuana [72% smoking, 47% daily use] and 21% were using CBD, usually for relieving pain [85%] and anxiety [31%]; the effects of liver tests and transplant outcomes could not be assessed).
- Ballotin VR, Bigarella LG, Brandão ABM, Balbinot RA, Balbinot SS, Soldera J. Herb-induced liver injury: systematic review and meta-analysis. World J Clin Cases. 2021;9:5490–5513. [PMC free article: PMC8281430] [PubMed: 34307603](Systematic review of the literature on herb induced liver injury identified 446 references describing 936 cases of liver injury due to 79 different herbal products, the most common being He Shou Wu [n=91], green tea [90] Herbalife products [64], kava kava [62] and greater celandine [48]; cannabis and marijuana were no listed).
- Abosheaishaa H, Nassar M, Haseeb Ul Rasool M, Makhoul K, Abdelwahed M. Marijuana-induced acute hepatitis: a case report. Cureus. 2022;14:e30273. [PMC free article: PMC9653216] [PubMed: 36381892](34 year old African American woman developed nausea and vomiting and was found to have abnormal liver tests [bilirubin 0.6 mg/dL, ALT 411 U/L, AST 2636 U/L, Alk P 411 U/L, INR 1.1] and a history of marijuana use and positive urine screening test for THC, with no other obvious cause of the liver injury).
- Abelev S, Warne LN, Benson M, Hardy M, Nayee S, Barlow J. Medicinal cannabis for the treatment of chronic refractory pain: an investigation of the adverse event profile and health-related quality of life impact of an oral formulation. Med Cannabis Cannabinoids. 2022;5:20–31. [PMC free article: PMC9235063] [PubMed: 35950052](Among 151 adults with chronic pain treated with an oil-based mixture of THC and CBD, pain scores improved minimally and 60% of patients had adverse events, none of which were serious; no mention of ALT elevations or hepatotoxicity).
- EFSA Panel on Nutrition. Statement on safety of cannabidiol as a novel food: data gaps and uncertainties. EFSA J. 2022;20:e07322. Novel Foods and Food Allergens (NDA), Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Maciuk A, Mangelsdorf I, et al. [PMC free article: PMC9172591] [PubMed: 35686177](Major review of the mechanisms of action, clinical effects and toxicities of cannabidiol [CBD] used as a “novel food” in the European Union, difficulties being the variable purity and concentration of commercial CBD products, its uncertain mechanism of action, the lack of rigorous clinical studies of its efficacy, and poor description of potential toxicities leading to the conclusion that more and better studies are needed; in animal models CBD increases the relative liver weight and has variably been found to result in transient elevations in ALT, Alk P and bilirubin; similar results have been found in healthy human volunteers, but largely in higher doses and without instances of clinically apparent liver injury).
- Nathan R, Mupamombe CT, Elibol J, Case AA, Smith D, Hyland A, Attwood K, et al. Assessing efficacy and use patterns of medical cannabis for symptom management in elderly cancer patients. Am J Hosp Palliat Care. 2022:10499091221110217. [PubMed: 35749740](Among 85 cancer patients ages 65 years and above who were treated with medical cannabis products [containing both THC and CBD] who filled out symptom questionnaires before and during therapy, there were slight decreases but not statistically significant changes in pain, nausea, anorexia or anxiety; no mention of adverse events, ALT elevations or hepatotoxicity).
- Englund A, Oliver D, Chesney E, Chester L, Wilson J, Sovi S, De Micheli A, et al. Does cannabidiol make cannabis safer? A randomised, double-blind, cross-over trial of cannabis with four different CBD:THC ratios. Neuropsychopharmacology. 2022 Nov 16; Epub ahead of print. [PMC free article: PMC10156730] [PubMed: 36380220](Among 47 healthy volunteers given granulated cannabis after pretreatment with one of 3 doses of cannabidiol [20, 30 or 40 mg] or placebo, serum THC concentrations and its cognitive and psychological effects were not affected by CBD pretreatment).
- Stolar O, Hazan A, Vissoker RE, Kishk IA, Barchel D, Lezinger M, Dagan A, et al. Medical cannabis for the treatment of comorbid symptoms in children with autism spectrum disorder: An interim analysis of biochemical safety. Front Pharmacol. 2022 Sep 29;13:977484. [PMC free article: PMC9559854] [PubMed: 36249785](Among 59 children with autistic spectrum disorder treated with cannabidiol in medium chain triglyceride oil in escalating doses for an average of 18 weeks, there were no clinical or statistically significant changes in complete blood counts or chemistry tests including ALT, AST, Alk P).
- Pinazo-Bandera JM, García-Cortés M, Segovia-Zafra A, Lucena MI, Andrade RJ. Recreational drugs and the risk of hepatocellular carcinoma. Cancers (Basel). 2022;14:5395. [PMC free article: PMC9657889] [PubMed: 36358813](Review of the association of hepatocellular carcinoma with recreational drug use, including Khat, kava kava, cannabis, cigarette smoking, cocaine, heroin, amphetamines and anabolic steroids, states that the association of marijuana use with liver cancer is controversial and meta-analyses have concluded that cannabis use is not associated with worsening of liver fibrosis).
- Barré T, Bourlière M, Ramier C, Carrat F, Di Beo V, Protopopescu C, Marcellin F, et al. Anrs/Afef Hepather Study Group. Cannabis use is inversely associated with metabolic disorders in hepatitis C-infected patients (ANRS CO22 Hepather Cohort). J Clin Med. 2022;11:6135. [PMC free article: PMC9605108] [PubMed: 36294456](Among 6464 adults with chronic hepatitis C enrolled in a French national cohort study, 12% were current users and 20% former users of marijuana, and in multivariate analysis, the cannabis users were less likely to have hypertension and multiple metabolic conditions such as obesity and diabetes, suggesting that marijuana use is protective against metabolic dysregulation).
- Kanjanarangsichai A, Mitarnun W, Mitarnun W, Pangwong W, Laoharattanahirun N, Kajornrith W, Junlaor P, et al. Cannabidiol-enriched cannabis extraction product in Parkinson's disease: a randomized, double-blind, and placebo-controlled trial in Buriram Hospital. J Neurosci Rural Pract. 2022;13:663–668. [PMC free article: PMC9894020] [PubMed: 36743777](Among 40 patients with Parkinson disease treated with a sublingual cannabinoid product [16 mg of cannabidiol and 0.6 mg of THC daily] for 8 weeks, there were no differences in measures of Parkinson disease symptoms and signs and no differences in serum ALT, AST, Alk P and bilirubin levels between the two groups).
- Bessone F, García-Cortés M, Medina-Caliz I, Hernandez N, Parana R, Mendizabal M, Schinoni MI, et al. Herbal and dietary supplements-induced liver injury in Latin America: experience from the LATINDILI Network. Clin Gastroenterol Hepatol. 2022;20:e548–e563. [PubMed: 33434654](Among 367 cases of hepatotoxicity enrolled in the Latin American DILI Network between 2011 and 2019, 29 [8%] were attributed to herbal products, the most frequent being green tea [n=7], Herbalife products [n=5] and garcinia [n=3], while marijuana and cannabis were not implicated in any cases).
- Pillai M, Erridge S, Bapir L, Nicholas M, Dalavaye N, Holvey C, Coomber R, et al. Assessment of clinical outcomes in patients with post-traumatic stress disorder: analysis from the UK Medical Cannabis Registry. Expert Rev Neurother. 2022;22(11-12):1009–1018. [PubMed: 36503404](Among 162 patients enrolled in the UK Medical Cannabis Registry who were treated for post-traumatic stress disorder, improvements in PTSD symptoms occurred with therapy and adverse events were reported in 20% of patients including fatigue, insomnia and dizziness but with no reports of liver injury).
- Vickery AW, Roth S, Ernenwein T, Kennedy J, Washer P. A large Australian longitudinal cohort registry demonstrates sustained safety and efficacy of oral medicinal cannabis for at least two years. PLoS One. 2022;17:e0272241. [PMC free article: PMC9674134] [PubMed: 36399463](Among 3961 patients in an Australian longitudinal registry of patients treated with medicinal cannabis for at least 2 years, improvements in symptoms were rapid and sustained for two, during which 37% had at least one adverse event including somnolence [11%], dry mouth [9%], lethargy [6%], dizziness [6%] and nausea [5%], without mention of liver related adverse events or ALT elevations).
- Gelow K, Chalasani S, Green K, Lammert C. Utilization and impact of complementary and alternative medicines in symptomatic autoimmune hepatitis patients. Dig Dis Sci. 2022;67:2891–2898. [PMC free article: PMC9236966] [PubMed: 34160734](Among patients with autoimmune hepatitis who completed an on-line questionnaire, 45% reported using CBD oil after the diagnosis was made usually for fatigue, pain, disturbed sleep, or itching).
- Olsson F, Erridge S, Tait J, Holvey C, Coomber R, Beri S, Hoare J, et al. An observational study of safety and clinical outcome measures across patient groups in the United Kingdom Medical Cannabis Registry. Expert Rev Clin Pharmacol. 2023;16:257–266. [PubMed: 36848456](Among 2833 persons enrolled in the UK Medical Cannabis Registry between 2019 and 2022, indications for treatment included non-cancer pain [32%], anxiety [11%], fibromyalgia [11%], neuropathy [8%]. PTSD [6%] and depression [5%], health related quality of life improved after 1 month and was improved in all subscales except self-care at 12 months, the most common therapies were THC oil [20 mg/mL] and CBD oil [50 mg/mL]; adverse events being reported by 17% of patients which were usually mild or moderate [79%], most commonly fatigue [14%], dry mouth [12%], somnolence [11%] insomnia [11%] headache [10%], impaired concentration [10%], nausea [9%], and dizziness [8%]; no mention of ALT elevations or liver injury).
- Morris M, Chye R, Liu Z, Agar M, Razmovski-Naumovski V. A retrospective medical record review of adults with non-cancer diagnoses prescribed medicinal cannabis. J Clin Med. 2023;12:1483. [PMC free article: PMC9965412] [PubMed: 36836018](Among 157 adults treated with medicinal cannabis [typically balanced THC/CBD oil] at a single specialty clinic after its approval in Australia, the major reasons were for pain [87%], muscle spasm [12%] and sleep [6%] and its was considered beneficial by the patient in 54%, most frequently for neuropathy [67%], Parkinson disease [61%], multiple sclerosis [60%], migraine [44%], chronic pain syndrome [42% and spondylosis [40%], and specifically for sleep [80%], fatigue [52%], and pain [50%]; side effects were reported in 43% of patients and were generally mild including somnolence, dry mouth and confusion; no mention of ALT elevations or liver injury).
- Waissengrin B, Leshem Y, Taya M, Meiri D, Merimsky O, Shamai S, Wolf I, Rubinek T. The use of medical cannabis concomitantly with immune checkpoint inhibitors in non-small cell lung cancer: A sigh of relief? Eur J Cancer. 2023;180:52–61. [PubMed: 36535195](Among 201 adults with metastatic non-small cell lung cancer treated with pembrolizumab as a first line therapy, 51% were prescribed cannabis products for cancer symptoms [mostly pain and poor appetite], but time to tumor progression was similar with or without cannabis therapy and overall survival was numerically but not statistically better in the cannabis treated subjects; no mention of side effects, ALT levels or hepatotoxicity).
- Review Cannabidiol.[LiverTox: Clinical and Researc...]Review Cannabidiol.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Wonder or evil?: Multifaceted health hazards and health benefits of Cannabis sativa and its phytochemicals.[Saudi J Biol Sci. 2021]Review Wonder or evil?: Multifaceted health hazards and health benefits of Cannabis sativa and its phytochemicals.Datta S, Ramamurthy PC, Anand U, Singh S, Singh A, Dhanjal DS, Dhaka V, Kumar S, Kapoor D, Nandy S, et al. Saudi J Biol Sci. 2021 Dec; 28(12):7290-7313. Epub 2021 Aug 19.
- Review [Marijuana--2000].[Orv Hetil. 2001]Review [Marijuana--2000].Bálint GS. Orv Hetil. 2001 Apr 15; 142(15):771-3.
- Review Cannabis and the exocannabinoid and endocannabinoid systems. Their use and controversies.[Gac Med Mex. 2019]Review Cannabis and the exocannabinoid and endocannabinoid systems. Their use and controversies.Millán-Guerrero RO, Isais-Millán S. Gac Med Mex. 2019; 155(5):471-474.
- Characterization of new chloroplast markers to determine biogeographical origin and crop type of Cannabis sativa.[Int J Legal Med. 2019]Characterization of new chloroplast markers to determine biogeographical origin and crop type of Cannabis sativa.Roman MG, Gangitano D, Houston R. Int J Legal Med. 2019 Nov; 133(6):1721-1732. Epub 2019 Aug 23.
- Marijuana - LiverToxMarijuana - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...