NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
L-glutamine is an essential amino acid and precursor of major intracellular antioxidant molecules that is used in high doses to prevent vaso-occlusive crises in patients with sickle cell disease. L-glutamine has not been associated with serum enzyme elevations during therapy or to instances of idiosyncratic acute liver injury.
Background
L-glutamine (el gloo’ ta mene) is a conditionally essential amino acid that is approved for use in the treatment of sickle cell disease to prevent painful crises. Sickle cell disease is caused by an inherited mutation in the β globin gene that creates hemoglobin S, an abnormal form of hemoglobin which is prone to polymerization with deoxygenation resulting in sickling of red blood cells, hemolytic anemia, vascular occlusion of small vessels causing ischemic tissue and organ injury and recurrent painful crises. Sickle cell disease affects at least 100,000 Americans and is most common in persons of African descent. Long term complications include disability due to recurrent painful crises, acute chest syndrome, pulmonary hypertension, stroke and cerebral infracts, end-organ damage and early mortality. L-glutamine appears to act in decreasing painful crises in sickle cell disease by increasing levels of nicotinamide adenine dinucleotide (NAD) and glutathione, essential molecules in intracellular oxidative-reductive balance and metabolism. Sickled red cells appear to have decreased levels of reduced NAD and are susceptible to oxidative stress. Oral administration of L-glutamine can restore NAD levels towards normal. In a single, randomized controlled trial in patients with frequent sickle cell crises, high oral doses of pharmaceutic grade L-glutamine was associated with a decrease in the number of pain crises and hospitalizations, but had no effects on hemoglobin levels or reticulocyte counts. Based upon that single study, L-glutamine was approved in the United States in 2019 as therapy for sickle cell disease in adults and children 5 years or older. L-glutamine is available in powdered form in packets of 5 grams under the brand name Endari. The recommended dose, mixed with 8 oz. of beverage, varies by body weight, with twice daily doses of 5 grams if <30 kg, 10 grams if 30-65 kg, and 15 grams if >65 kg. Side effects can include fatigue, muscle and back pain, headache, abdominal pain, nausea, constipation, cough and non-cardiac chest pain.
Hepatotoxicity
In clinical trials of L-glutamine in patients with sickle cell disease, serum aminotransferase elevations were not mentioned, and there were no reports of clinically apparent liver injury. Patients with sickle cell disease frequently have jaundice, largely due to chronic hemolysis which raises serum indirect bilirubin levels. They also can have fluctuating liver test abnormalities due to complications of sickle cell disease, such as gall stone disease (from chronic hemolysis), viral hepatitis and iron overload (from blood transfusions), congestive liver disease (due to pulmonary hypertension), and veno-occlusive crises involving the liver which can be associated with serum aminotransferase elevations and hepatic dysfunction. In preregistration trials of L-glutamine, hepatic events were not reported and serious adverse events were no more common with the active drug than with placebo. L-glutamine is a normal constituent of virtually all tissues and is unlikely to have intrinsic toxicity, even in high doses.
Glutamine supplementation has a potential of worsening hepatic encephalopathy in patients with advanced cirrhosis. Glutamine is metabolized to glutamate and ammonia which can overwhelm the hepatic elimination of ammonia in patients with severe liver dysfunction. Ingestion of 10 to 20 grams of glutamine has been shown to cause elevations of serum ammonia levels and to worsen psychometric measures of hepatic encephalopathy in patients with decompensated cirrhosis. Plasma ammonia levels do not increase with glutamine supplementation in patients with normal hepatic function, and its effects in patients with cirrhosis is not due to hepatic injury. Nevertheless, use of L-glutamine should be avoided in patients with sickle cell disease and advanced cirrhosis.
Likelihood score: E (unlikely cause of acute liver injury with jaundice).
Mechanism of Injury
The mechanism by which L-glutamine might cause liver injury is unknown. L-glutamine is an amino acid that is used in protein synthesis in virtually all tissues and organs.
Outcome and Management
Elucidating the cause of liver test abnormalities in patients with sickle cell disease on therapies to prevent vaso-occlusive crises is difficult, as they are susceptible to several forms of liver injury including acute viral hepatitis, hemosiderosis, gallstone disease, congestive hepatopathy and ischemic liver injury from hepatic sickling crises.
Drug Class: Genetic Disorder Agents, Hematologic Agents, Sickle Cell Disease Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
L-Glutamine – Endari®
DRUG CLASS
Sickle Cell Disease Agents
Product labeling at DailyMed, National Library of Medicine, NIH
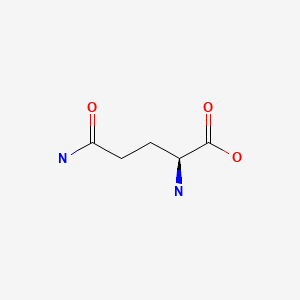
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| L-glutamine | 56-85-9 | C5-H10-N2-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 12 July 2021
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the reported use of L-glutamine in sickle cell disease).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2017/208587Orig1s000MedR.pdf. (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA multidisciplinary scientific review of the L-glutamine application for safety and efficacy which mentions that there were “no notable differences in clinical chemistry” parameters between treatment and placebo groups). - Oppong KN, Al-Mardini H, Thick M, Record CO. Oral glutamine challenge in cirrhotics pre- and post-liver transplantation: a psychometric and analyzed EEG study. Hepatology. 1997;26:870–6. [PubMed: 9328307]
- (Among 17 patients with cirrhosis and 4 normal controls given oral challenges with 10 or 20 gm of glutamine, venous ammonia levels increased in those with cirrhosis [mean 58 to 120 µmol/L] but not in controls [mean 32 to 39 µmol/L], and several patients with cirrhosis had worsening of psychometric and EEG measures of subclinical hepatic encephalopathy) .
- Holecek M. Side effects of long-term glutamine supplementation. JPEN J Parenter Enteral Nutr. 2013;37:607–16. [PubMed: 22990615]
- (Review of the potential adverse events of long term administration of glutamine supplementation in parenteral nutrition mentions that the metabolism of glutamine to glutamate generates ammonia which may overwhelm or circumvent hepatic metabolism) .
- Koh C, Turner T, Zhao X, Minniti CP, Feld JJ, Simpson J, Demino M, et al. Liver stiffness increases acutely during sickle cell vaso-occlusive crisis. Am J Hematol. 2013;88:E250–4. [PMC free article: PMC3808506] [PubMed: 23828202](Among 23 patients with sickle cell disease evaluated before and during an acute vaso-occlusive crisis, serum liver enzyme elevations did not change appreciably but hepatic stiffness increased [measured by ultrasound transient elastography] as did serum total and indirect bilirubin and reticulocyte counts, while serum albumin and hemoglobin decreased).
- Feld JJ, Kato GJ, Koh C, Shields T, Hildesheim M, Kleiner DE, Taylor JG 6th, et al. Liver injury is associated with mortality in sickle cell disease. Aliment Pharmacol Ther. 2015;42:912–21. [PMC free article: PMC6478018] [PubMed: 26235444](Among 247 patients with sickle cell disease, liver disease was common, elevations in ALT were present in 16% and alkaline phosphatase in 33%; factors associated with mortality during follow up were iron indices [serum ferritin, transferrin, and iron] and liver abnormalities [direct bilirubin, albumin and alkaline phosphatase levels]; liver biopsy done in 40 patients revealed nodular regenerative hyperplasia in 36% and portal venopathy in 23%).
- L-glutamine (Endari) for sickle cell disease. Med Lett Drugs Ther. 2018;60(1539):21–2. [PubMed: 29364198](Concise review of the mechanism of action, clinical efficacy, safety and cost of L-glutamine for sickle cell disease describes its adverse effects to include constipation, nausea, headache, abdominal pain, pain in back and extremities, cough and chest pain but does not mention ALT elevations or hepatotoxicity).
- Quinn CT. L-glutamine for sickle cell anemia: more questions than answers. Blood. 2018;132:689–93. [PubMed: 29895661](Review of the evolving understanding of sickle cell disease and the pathogenesis of the painful crises focusing upon studies of oxidative stress and the possible role of L-glutamine in protecting red cells from oxidative damage; mentions the modest clinical effects of L-glutamine therapy on sickle cell crises, the lack of understanding of mechanism of action, its high cost, and possibly poor tolerance).
- Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, Gordeuk VR, et al. Investigators of the Phase 3 Trial of l-Glutamine in Sickle Cell Disease. A phase 3 trial of L-glutamine in sickle cell disease. N Engl J Med. 2018;379:226–35. [PubMed: 30021096](Among 230 patients with sickle cell disease, ages 5 to 58 years, treated with L-glutamine [0.3 gm/kg] or placebo twice daily for 48 weeks, painful crises were less frequent with L-glutamine that placebo [mean 3.2 vs 3.9 crises per year], while both total and severe adverse event rates were similar in the two groups; no mention of ALT elevations or hepatotoxicity).
- Osunkwo I, Manwani D, Kanter J. Current and novel therapies for the prevention of vaso-occlusive crisis in sickle cell disease. Ther Adv Hematol. 2020;11:2040620720955000. [PMC free article: PMC7534097] [PubMed: 33062233](Extensive review of the pathogenesis of sickle cell vaso-occlusive crises and potential therapeutic avenues for their prevention and treatment including induction of hemoglobin F [hydroxyurea], decreasing oxidative stress [L-glutamine], decreasing red cell adhesion [crizanlizumab], and inhibition of hemoglobin S polymerization).
- Darbari DS, Sheehan VA, Ballas SK. The vaso-occlusive pain crisis in sickle cell disease: definition, pathophysiology, and management. Eur J Haematol. 2020;105:237–46. [PubMed: 32301178](Extensive review of the pathogenesis of vaso-occlusive crisis in patients with sickle cell disease and therapies that target different steps in the process including inflammation, adhesion, oxidative stress, and oxygen affinity and stability of hemoglobin; discusses efficacy of L-glutamine, voxelotor and crizanlizumab, mentioning that all three are well tolerated; no mention or discussion of hepatotoxicity).
- Ali MA, Ahmad A, Chaudry H, Aiman W, Aamir S, Anwar MY, Khan A. Efficacy and safety of recently approved drugs for sickle cell disease: a review of clinical trials. Exp Hematol. 2020;92:11–18.e1. [PMC free article: PMC7442900] [PubMed: 32841705](Review of randomized controlled trials of 3 recently approved drugs for sickle cell disease focusing upon L-glutamine, voxelotor, and crizanlizumab states that all three are “well tolerated without any alarming adverse effects”; no mention of ALT elevations or hepatotoxicity).
- Pace BS, Starlard-Davenport A, Kutlar A. Sickle cell disease: progress towards combination drug therapy. Br J Haematol. 2021;194(2):240–51. [PMC free article: PMC8282668] [PubMed: 33471938](Review of the pathophysiology of sickle cell disease and vaso-occlusive crises and mechanism of action of drugs used to treat sickle cell disease and drugs currently under investigation for efficacy in decreasing the microvascular occlusive crises that mediate much of the morbidity and mortality of this disease).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Contemporary Management and Prevention of Vaso-Occlusive Crises (VOCs) in Adults With Sickle Cell Disease.[J Pharm Pract. 2023]Review Contemporary Management and Prevention of Vaso-Occlusive Crises (VOCs) in Adults With Sickle Cell Disease.Weaver SB, Rungkitwattanakul D, Singh D. J Pharm Pract. 2023 Feb; 36(1):139-148. Epub 2021 Jun 21.
- Review Comparing the Safety and Efficacy of L-Glutamine, Voxelotor, and Crizanlizumab for Reducing the Frequency of Vaso-Occlusive Crisis in Sickle Cell Disease: A Systematic Review.[Cureus. 2022]Review Comparing the Safety and Efficacy of L-Glutamine, Voxelotor, and Crizanlizumab for Reducing the Frequency of Vaso-Occlusive Crisis in Sickle Cell Disease: A Systematic Review.Dick MH, Abdelgadir A, Kulkarni VV, Akram H, Chatterjee A, Pokhrel S, Khan S. Cureus. 2022 May; 14(5):e24920. Epub 2022 May 11.
- Systematic Review of l-glutamine for Prevention of Vaso-occlusive Pain Crisis in Patients with Sickle Cell Disease.[Pharmacotherapy. 2019]Systematic Review of l-glutamine for Prevention of Vaso-occlusive Pain Crisis in Patients with Sickle Cell Disease.Cieri-Hutcherson NE, Hutcherson TC, Conway-Habes EE, Burns BN, White NA. Pharmacotherapy. 2019 Nov; 39(11):1095-1104. Epub 2019 Oct 9.
- Review L-glutamine for sickle cell disease: more than reducing redox.[Ann Hematol. 2022]Review L-glutamine for sickle cell disease: more than reducing redox.Jafri F, Seong G, Jang T, Cimpeanu E, Poplawska M, Dutta D, Lim SH. Ann Hematol. 2022 Aug; 101(8):1645-1654. Epub 2022 May 14.
- Magnetic Resonance Imaging Assessment of Kidney Oxygenation and Perfusion During Sickle Cell Vaso-occlusive Crises.[Am J Kidney Dis. 2017]Magnetic Resonance Imaging Assessment of Kidney Oxygenation and Perfusion During Sickle Cell Vaso-occlusive Crises.Deux JF, Audard V, Brugières P, Habibi A, Manea EM, Guillaud-Danis C, Godeau B, Galactéros F, Stehlé T, Lang P, et al. Am J Kidney Dis. 2017 Jan; 69(1):51-59. Epub 2016 Sep 20.
- L-Glutamine - LiverToxL-Glutamine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...