NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ketorolac is a potent, short acting nonsteroidal antiinflammatory drug (NSAID) that is available in both parenteral and oral forms. Ketorolac is generally given for a few days only, and has not been linked to instances of idiosyncratic drug induced liver disease in the published literature.
Background
Ketorolac tromethamine belongs to the acetic acid class of NSAIDs similar to diclofenac and etodolac. Like other NSAIDs, ketorolac is a potent cyclo-oxygenase (Cox) inhibitor which blocks the formation of prostaglandins that are important in pain and inflammatory pathways. Ketorolac was approved in the United States in 1991 and current indications are limited to the short term management of moderately severe, acute pain. Ketorolac is available in parenteral and oral forms in multiple generic forms and under the brand name Toradol. The recommended dose is 60 mg intramuscularly or 30 mg intravenously initially, followed by 30 mg every 6 hours for up to 5 days. An oral form is available in 10 mg tablets for switching from the parenteral form and is given every 6 to 8 hours, but continuation beyond 5 days is not recommended. Ketorolac is available by prescription only and it is used largely for management of postoperative pain. Common side effects include gastrointestinal upset, nausea, headache and itching.
Hepatotoxicity
Prospective studies show that up to 1% of patients taking ketorolac experience at least transient serum aminotransferase elevations. These may resolve even with drug continuation. Marked aminotransferase elevations (>3 fold elevated) occur in <1% of patients. Clinically apparent liver injury with jaundice from ketorolac has not been reported so that the latency, clinical features and prognosis of injury is unknown. Ketorolac is not mentioned as an etiologic agent in large case series on drug induced liver injury or acute liver failure. However, the antiplatelet activity of ketorolac can lead to complications during relief of postoperative pain, and several instances of hepatic rupture and subcapsular hepatic hematomas have been reported with its use.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury, largely due to bleeding episodes).
Mechanism of Injury
The mechanism of ketorolac hepatotoxicity, if it exists, is not known. The hepatic hematomas described after its use are probably caused by the antiplatelet activity of ketorolac given in the perioperative period.
Outcome and Management
Only asymptomatic elevations in serum aminotransferase levels have been reported associated with ketorolac therapy. The drug is rarely used outside of hospitals and it is recommended that it be used for no more than 5 days, perhaps accounting for the rarity or absence of hepatic injury.
Drug Class: Nonsteroidal Antiinflammatory Drugs
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ketorolac – Generic, Toradol®
DRUG CLASS
Nonsteroidal Antiinflammatory Drugs
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
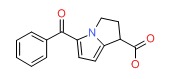
| Ketorolac | 74103-06-3 | C15-H13-N-O3 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 25 April 2018
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. The NSAIDS. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 517-41.(Review of hepatotoxicity of NSAIDs published in 1999; ketorolac is not mentioned).
- Lewis JH, Stine JG. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists: pathology and clinical presentation of hepatotoxicity. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. pp. 370-402.(Review of hepatotoxicity of NSAIDs mentions that ketorolac has not been implicated in cases of clinically apparent liver injury in the published literature).
- Grossner T, Smyth EM, Fitzgerald GA. Anti-inflammatory, antipyretic, and analgesic agents: pharmacotherapy of gout. In, Brunton LL, Chabner BA, Knollman BC. Goodman & Gilman’s The pharmacological basis of therapeutics, 12th ed. New York: McGraw-Hill, 2011. p. 959-1004.(Textbook of pharmacology and therapeutics).
- Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet 1992; 23: 415-27. [PubMed: 1458761](Ketorolac is produced as a tromethamine salt to allow water solubility and parenteral administration, is rapidly absorbed, has a half life of 4-6 hours, is highly protein bound, extensively conjugated in the liver and excreted in urine).
- Erstad BL, Rappaport WD. Subcapsular hematoma after laparoscopic cholecystectomy, associated with ketorolac administration. Pharmacotherapy 1994; 14: 613-5. [PubMed: 7997396](35 year old woman developed subcapsular hepatic hematoma 10 hours after after receving intravenous ketorolac after laparoscopic cholecystectomy).
- Hennessy S, Kinman JL, Berlin JA, Feldman HI, Carson JL, Kimmel SE, et al. Lack of hepatotoxic effects of parenteral ketorolac in the hospital setting. Arch Intern Med 1997; 157: 2510-4. [PubMed: 9385304](Analysis of safety based upon 10,272 courses of ketorolac given to 9900 patients in 35 hospitals, average duration of therapy was for 2.6 days [range 1-44]; ALT elevations >3 fold occurred in 0.7% of ketorolac vs 0.9% of opioid courses).
- Zimmerman HJ. Update of hepatotoxicity due to classes of drugs in common clinical use: non-steroid drugs, anti-inflammatory drugs, antibiotics, antihypertensives, and cardiac and psychotropic agents. Semin Liver Dis 1990; 10: 322-8. [PubMed: 2281340](Extensive review article on liver injury due to NSAIDs; ketorolac is not discussed).
- Vuilleumier H, Halkic N. Ruptured subcapsular hematoma after laparoscopic cholecystectomy attributed to ketorolac-induced coagulopathy. Surg Endosc 2003; 17: 659. [PubMed: 12574932](23 year old woman received ketorolac after routine laparoscopic cholecystectomy and presented 18 hours later with subcapsular hematoma of the liver attributed to platelet effects of ketorolac).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](Survey of all cases of drug induced liver injury with fatal outcome from Swedish Adverse Drug Reporting system from 1966-2002; among 103 cases, 3 attributed to naproxen, but none to ketorolac).
- Lapeyre-Mestre M, de Castro AM, Bareille MP, Del Pozo JG, Requejo AA, Arias LM, et al. Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. Fundam Clin Pharmacol 2006; 20:391-5. [PubMed: 16867024](Analysis of reports of liver injury from NSAIDs from France and Spain from 1982-2001; among more than 29,000 liver adverse event reports, 11 were for ketorolac; no clinical details given; ketorolac withdrawn from France in 1994 because of gastrointestinal side effects).
- Guercio G, Sandonato L, Cintorino D, Ricotta C, Diana G. [Hemoperitoneum from rupture of liver subcapsular hematoma after laparoscopic cholecystectomy attributed to ketorolac. Report of a case]. G Chir 2008; 29: 351-3. Italian. [PubMed: 18834567](39 year old woman undergoing laparoscopic cholecystectomy received intravenous ketorolac postoperatively and developed a subcapsular hepatic hematoma 7 hours later).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, NSAIDs were implicated as a sole agent in 8 cases [4 diclofenac, 2 celecoxib, 1 meloxicam and 1 oxaprozin] and as one of several agents in 3 cases [1 diclofenac, 1 celecoxib, 1 ibuprofen]; none were attributed to ketorolac).
- Minaya Bravo AM, González González E, Ortíz Aguilar M, Larrañaga Barrera E. Two rare cases of intrahepatic subcapsular hematoma after laparoscopic cholecystectomy. Indian J Surg 2010; 72: 481-4. [PMC free article: PMC3077204] [PubMed: 22131659](Two women, ages 29 and 69 years, developed symptomatic subcapsular hepatic hematomas 1 and 5 days after laparoscopic cholecystectomy while receiving intravenous ketorolac for pain; both required surgery and recovered uneventfully).
- Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol 2010; 16: 5651-61. [PMC free article: PMC2997980] [PubMed: 21128314](Review of estimated frequency of drug induced liver injury due to NSAIDs from large published epidemiological studies; ketorolac is not discussed).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which 7 were attributed to NSAIDs including 4 to bromfenac, 2 diclofenac and 1 etodolac, but none to ketorolac).
- Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, Jové J, Gatta A, et al. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case-population SALT study. Drug Saf 2013; 36: 135-44. [PMC free article: PMC3568201] [PubMed: 23325533](Among 600 patients undergoing liver transplantation for acute liver failure at 52 European liver transplant centers between 2005 and 2007, 301 were considered idiopathic and had received a medication within 30 days of onset, including acetaminophen in 192 and NSAIDs in 40, including ketorolac in 2 for a rate of 19.5 per million-treatment years).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. (In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 6 attributed to diclofenac [ranking 2nd], but none for ketorolac or other NSAIDs). [PubMed: 23419359]
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common class of implicated agents being NSAIDS [n=62, 32%], but specific agents were nimesulide [n=53], piroxicam [5], diclofenac [2], gold salts [1], and naproxen [1]; ketorolac was not listed]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 28 were attributed to NSAIDs, but none to ketorolac [Schmeltzer 2016]).
- Schmeltzer PA, Kosinski AS, Kleiner DE, Hoofnagle JH, Stolz A, Fontana RJ, Russo MW; Drug-Induced Liver Injury Network (DILIN). Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int 2016; 36: 603-9. [PMC free article: PMC5035108] [PubMed: 26601797](Among 1221 cases of drug induced liver injury enrolled in a prospective, US database between 2004 and 2014, 30 cases [2.5%] were attributed to NSAIDs, most common diclofenac; none were attributed to ketorolac).
- Donati M, Conforti A, Lenti MC, Capuano A, Bortolami O, Motola D, Moretti U, et al; DILI-IT Study Group. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy. Br J Clin Pharmacol 2016; 82: 238-48. [PMC free article: PMC4917796] [PubMed: 26991794](Among 179 cases of acute liver injury and 1770 controls admitted to 9 Italian hospitals between 2010 and 2014, NSAIDs used more frequently in cases compared to controls included nimesulide [17% vs 10%: odds ratio 1.88] and ibuprofen [14% vs 10%: odds ratio 1.59] and risk was higher in those taking higher doses; ketorolac was not mentioned).
- Zoubek ME, González-Jimenez A, Medina-Cáliz I, Robles-Díaz M, Hernandez N, Romero-Gómez M, Bessone F, et al. High Prevalence of ibuprofen drug-induced Liver injury in Spanish and Latin-American registries. Clin Gastroenterol Hepatol 2018; 16: 292-4. [PubMed: 28782674](Analysis of a Spanish and Latin-American registries identified 73 cases of NSAID induced liver injury, the most common agents being nimesulide [38%], diclofenac [34%] and ibuprofen [17%]; ketorolac was not mentioned).
- Maslin B, Lipana L, Roth B, Kodumudi G, Vadivelu N. Safety considerations in the use of ketorolac for postoperative pain. Curr Drug Saf 2017; 12: 67-73. PubMed Citation. [PubMed: 27440142](Review of the safety of ketorolac in the perioperative period focusing upon renal dysfunction and bleeding; no discussion of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Ketorolac: a parenteral nonsteroidal antiinflammatory drug.[DICP. 1990]Review Ketorolac: a parenteral nonsteroidal antiinflammatory drug.Resman-Targoff BH. DICP. 1990 Nov; 24(11):1098-104.
- Review The use of ketorolac in the management of postoperative pain.[Orthopedics. 1994]Review The use of ketorolac in the management of postoperative pain.DeAndrade JR, Maslanka M, Maneatis T, Bynum L, Burchmore M. Orthopedics. 1994 Feb; 17(2):157-66.
- Ketorolac and patient controlled analgesia in the treatment of postoperative pain.[Surg Gynecol Obstet. 1993]Ketorolac and patient controlled analgesia in the treatment of postoperative pain.Cataldo PA, Senagore AJ, Kilbride MJ. Surg Gynecol Obstet. 1993 May; 176(5):435-8.
- Novel depots of ketorolac esters have long-acting antinociceptive and antiinflammatory effects.[Anesth Analg. 2005]Novel depots of ketorolac esters have long-acting antinociceptive and antiinflammatory effects.Liu SY, Shieh JP, Tzeng JI, Chia-Hui H, Cheng YL, Huang KL, Wang JJ. Anesth Analg. 2005 Sep; 101(3):785-792.
- Analgesic efficacy of ketorolac and morphine in neonatal rats.[Pharmacol Biochem Behav. 2001]Analgesic efficacy of ketorolac and morphine in neonatal rats.Gupta A, Cheng J, Wang S, Barr GA. Pharmacol Biochem Behav. 2001 Apr; 68(4):635-40.
- Ketorolac - LiverToxKetorolac - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...