NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ketoprofen is a nonsteroidal antiinflammatory drug (NSAID) used in treatment of acute pain and chronic arthritis. Ketoprofen has been linked to a low rate of serum enzyme elevations during therapy and to rare instances of clinically apparent acute liver injury.
Background
Ketoprofen (kee" toe proe' fen) belongs to the propionic derivative class of NSAIDs similar to naproxen and ibuprofen. Like other NSAIDs, ketoprofen is a cyclo-oxygenase (Cox-1 and -2) inhibitor that blocks the formation of prostaglandins that are important in pain and inflammatory pathways. Ketoprofen has analgesic as well as antipyretic and antiinflammatory activities. Ketoprofen was approved in the United States in 1986 and is still widely used. Current indications include chronic joint pain due to osteoarthritis and rheumatoid arthritis as well as mild-to-moderate acute pain and dysmenorrhea. The recommended dose in adults with chronic arthritis is 50 to 75 mg 3 or 4 times per day with a maximum dose of 300 mg daily. Ketoprofen is available by prescription in the form of capsules or tablets of 25, 50 and 75 mg in both generic and trade formulations (Orudis, Oruvail, among others). Extended release formulations of 100, 150 and 200 mg are also available for once daily dosing. Ketoprofen is also available in over-the-counter formulations of 12.5 mg tablets for treatment of mild-to-moderate pain and dysmenorrhea. As with other NSAIDs, ketoprofen is generally well tolerated, but side effects can include headache, dizziness, somnolence, gastrointestinal upset, nausea, abdominal discomfort, diarrhea, peripheral edema and hypersensitivity reactions.
Hepatotoxicity
Prospective studies show that 1% to 2% of patients taking ketoprofen experience at least transient serum aminotransferase elevations. These may resolve even with drug continuation. Marked aminotransferase elevations (>3 fold elevated) occur in <1% of patients. Clinically apparent liver injury with jaundice from ketoprofen is very rare and only individual case reports have been published. The latency to onset has been rapid, often within a few days of starting. The pattern of enzyme elevations has ranged from cholestatic to hepatocellular. Immunoallergic features are present in some cases (low grade fever, rash), but are generally not prominent, and autoantibody formation is rare. Most cases resolve promptly on stopping therapy. Ketoprofen is not mentioned in large case series on drug induced liver injury or acute liver failure.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of ketoprofen hepatotoxicity is not known, but likely to be due to an idiosyncratic reaction to an intermediate of its metabolism. Ketoprofen is extensively metabolized by the liver.
Outcome and Management
Severity ranges from asymptomatic elevations in serum aminotransferase levels, to symptomatic hepatitis with or without jaundice. A single case of fulminant hepatitis possibly due to ketoprofen has been reported, but death appeared to be due to complications of pancreatitis and renal insufficiency. Patients with ketoprofen induced liver injury should avoid other propionic acid derivatives such as naproxen and ibuprofen.
Drug Class: Nonsteroidal Antiinflammatory Drugs
CASE REPORTS
Case 1. Acute hepatocellular injury and jaundice arising within a few days of starting ketoprofen.
[Modified from: Bonaventure C, Nancey S, Pont E, Michalet V, Chevalier M, Vial T, Taieb S, et al. [Ketoprofen-induced acute hepatitis]. Gastroenterol Clin Biol 2001; 25: 716-7. French. PubMed Citation]
A 23 year old woman developed abdominal pain and nausea 2 days and jaundice 4 days after starting ketoprofen for low back pain. She had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. Her only other medication was birth control pills. Physical examination was normal except for jaundice; there was no fever, rash or hepatomegaly. Laboratory tests showed serum bilirubin of 2.9 mg/dL with marked elevations in serum aminotransferase levels (ALT~30 times ULN, AST~14 times ULN) and normal alkaline phosphatase (Table). There was no eosinophilia. Tests for hepatitis A, B, C and E were negative as were autoantibodies. A liver biopsy showed hepatic necrosis and inflammation, mild cholestasis and prominence of eosinophils in portal areas. Stopping ketoprofen was followed by rapid improvement. She remained on birth control pills.
Key Points
| Medication: | Ketoprofen (200 mg extended release once daily for 4 days) |
| Pattern: | Hepatocellular (R=30) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 2 days to symptoms, 4 days to jaundice |
| Recovery: | Complete recovery 2 months after stopping |
| Other medications: | Combination estrogen and progesterone birth control pill |
Laboratory Values
* ALT values were calculated based upon upper limit of normal values; bilirubin levels were converted from µmol/L to mg/dL.
Comment
The rapid appearance of hepatic injury suggests a hypersensitivity reaction, but most cases of ketoprofen induced hepatotoxicity have not been accompanied by fever, rash or eosinophilia. Whether there is cross reactivity with other NSAIDs of similar structure (ibuprofen, naproxen) is unknown. Ketoprofen is widely used and hepatotoxicity is exceedingly rare.
Case 2. Cholestatic liver injury arising within a few days of starting ketoprofen.
[Modified from: Rambaud S, Nores JM, Rémy JM. [Jaundice related to the ingestion of ketoprofen] Ann Med Interne (Paris) 1990; 141: 278. French. PubMed Citation]
A 49 year old man developed fever, malaise and abdominal pain after 2 days and jaundice after 4 days of ketoprofen therapy for renal colic. He had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. His other medications included aminopyrine and tiemonium which were started at the same time as ketoprofen. Physical examination was normal. Laboratory tests showed elevations in serum bilirubin with only mild increases in ALT and alkaline phosphatase (Table). There was no eosinophilia. Tests for hepatitis A, B, and C were negative as were routine autoantibodies. Ultrasound of the abdomen showed no abnormalities of the liver or biliary tract. Stopping ketoprofen was followed by rapid improvement.
Key Points
| Medication: | Ketoprofen (200 mg extended release once daily for 4 days) |
| Pattern: | Cholestatic (R=1.3) |
| Severity: | 3+ (jaundice, hospitalization prolonged) |
| Latency: | 2 days to symptoms, 4 days to jaundice |
| Recovery: | Complete recovery 2 months after stopping |
| Other medications: | Aminopyrine, tiemonium (antispasmodic) |
Laboratory Values
Comment
The similarity of onset to Case 1 is striking, but the enzyme pattern was quite different, with minimal increases in ALT and alkaline phosphatase rather than a hepatocellular pattern. These two cases suggest that latency to onset may be more reliable as a “signature” of drug induced liver injury than pattern of enzyme elevation.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ketoprofen – Generic, Orudis®, Oruvail®
DRUG CLASS
Antiinflammatory Agents, Nonsteroidal
Product labeling at DailyMed, National Library of Medicine, NIH
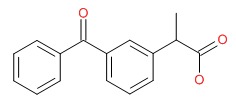
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ketoprofen | 22071-15-4 | C16-H14-O3 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 25 April 2018
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 517-53.(Expert review of hepatotoxicity published in 1999; ketoprofen is listed as having a very low rate of hepatic injury).
- Lewis JH, Stine JG. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists: pathology and clinical presentation of hepatotoxicity. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd. Amsterdam: Elsevier, 2013, pp. 369-401. (Review of hepatotoxicity of NSAIDs mentions that.a few cases of hepatotoxicity from ketoprofen have been reported).
- Grosser T, Smyth E, FitzGerald GA. Anti-inflammatory, antipyretic, and analgesic agents; pharmacotherapy of gout. In, Brunton LL, Chabner B, Knollman B, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 959-1004.(Textbook of pharmacology and therapeutics).
- Wollheim FA, Lindroth Y, Sjoblom KG. A comparison of ketoprofen and naproxen in rheumatoid arthritis. Rheumatol Rehabil 1978; Suppl: 78-83. [PubMed: 364615](Double-blind, cross over study of 4 week courses using different doses of ketoprofen vs naproxen in 30 patients with rheumatoid arthritis; common side effects were abdominal pain, dyspepsia, nausea, headache and pruritus; but "no abnormalities were found in... liver function tests").
- Gross W. Long-term studies on therapy and tolerance of ketoprofen. Rheumatol Rehabil 1978: Suppl: 112-3. [PubMed: 441625](Among 33 patients given ketoprofen and 32 given indomethacin for 5 months, analgesic effect was good with both and there were "no significant differences... on the parameters of liver or renal function").
- Llorca G, Larbre JP, Collet Ph, Ravault A, Lejeune E. Changing the class of NSAID in cases of hepatotoxicity. Ann Rheum Dis 1988; 47: 791. [PMC free article: PMC1003601] [PubMed: 3178321](44 year old man with ankylosing spondylitis developed mild Alk P elevations [144 U/L] and eosinophilia [9%] 2 months after starting diclofenac with normal ALT [9 U/L] and bilirubin [0.4 mg/dL]; abnormalities resolved rapidly on stopping diclofenac and did not reappear after switching to ketoprofen).
- Zimmerman HJ. Update of hepatotoxicity due to classes of drugs in common clinical use: non-steroid drugs, anti-inflammatory drugs, antibiotics, antihypertensives, and cardiac and psychotropic agents. Semin Liver Dis 1990; 10: 322-8. [PubMed: 2281340](Extensive review of NSAID related liver injury states that ketoprofen appears "not to have been incriminated in hepatic disease").
- Rambaud S, Nores JM, Rémy JM. [Jaundice related to the ingestion of ketoprofen] Ann Med Interne (Paris) 1990; 141: 278. French. [PubMed: 2369020](49 year old man developed symptoms after 2 and jaundice after 4 days of ketoprofen therapy [bilirubin 3.6 mg/dL, ALT 82 U/L, Alk P 186 U/L], resolving within 5 weeks of stopping: Case 2).
- Nores JM, Rambaud S, Remy JM. Acute hepatitis due to ketoprofen. Clin Rheumatol 1991; 10: 215-6. [PubMed: 1914425](Same case as in Rambaud [1990], but in English).
- Dutertre J-P, Bastides F, Jonville A-P, De Muret A, Sonneville A, Larrey D, Autret E. Microvesicular steatosis after ketoprofen administration. Eur J Gastroenterol Hepatol 1991; 3: 953-4. Not in PubMed.(47 year old man with rheumatoid arthritis developed abnormal Alk P [159 U/L] and GGT [145 U/L] without symptoms 2 weeks after starting ketoprofen that persisted and rose until ketoprofen was stopped 1 month later, resolving in 2 months; liver biopsy showed microvesicular steatosis).
- Walker AM. Quantitative studies of the risk of serious hepatic injury in persons using nonsteroidal antiinflammatory drugs. Arthritis Rheum 1997; 40: 201-8. [PubMed: 9041931](Review of population based studies of NSAID use and hepatic injury; frequency of clinically apparent liver injury from NSAIDs overall was ~10 cases per 100,000 patient-years of use; ketoprofen reported in one study as 17 per 100,000 [2 cases among 14,457 person-years of risk]).
- Flamenbaum M, Abergel A, Marcato N, Zénut M, Kémény JL, Cassan P. [Regressive fulminant hepatitis, acute pancreatitis and renal insufficiency after taking ketoprofen]. Gastroenterol Clin Biol 1998; 22: 975-6. [PubMed: 9881281](73 year old man developed vomiting and abdominal pain after 2 days and jaundice after 5 days of starting rectal ketoprofen [bilirubin 29.4 mg/dL, ALT 148 U/L, Alk P 1430 U/L], resolving upon stopping; patient also had pancreatitis and renal insufficiency; mental confusion was attributed to hepatic encephalopathy).
- Bonaventure C, Nancey S, Pont E, Michalet V, Chevalier M, Vial T, Taieb S, et al. [Ketoprofen-induced acute hepatitis] Gastroenterol Clin Biol 2001; 25: 716-7. French. [PubMed: 11673741](23 year old woman developed nausea after 2 days and jaundice after 4 days of ketoprofen therapy [bilirubin 2.9 mg/dL, ALT 30 times ULN, Alk P normal], resolving within 2 months of stopping: Case 1).
- Mété D, Milon A, Belon G, Gatina JH. [Acute pancreatitis and ketoprofen] Gastroenterol Clin Biol 2001; 25: 721-2. French. [PubMed: 11673744](35 year old man developed pancreatitis without jaundice or liver tests abnormalities 2 months after starting ketoprofen [lipase 1255 U/L], with no other cause identified and normal endoscopic retrograde cholangiopancreatography).
- Lacroix I, Lapeyre-Mestre M, Bagheri H, Pathak A, Montastruc JL; Club de Reflexion des cabinets de Groupe de Gastro-Enterologie (CREGG); General Practitioner Networks. Nonsteroidal anti-inflammatory drug-induced liver injury: a case-control study in primary care. Fundam Clin Pharmacol 2004; 18: 201-6. [PubMed: 15066135](Case controlled study of patients presenting with suspected drug induced liver injury in a general practice context in Southern France found 88 cases which were matched with 178 controls; 22 cases vs 16 controls had been exposed to NSAIDs; 5 diclofenac, 4 ibuprofen, 4 ketoprofen, 2 niflumic acid, 1 flurbiprofen and 1 meloxicam, rest to salicylates which were as frequently used in controls as cases; there were no fatalities and cases were more common in women than men).
- Rubenstein JH, Laine L. Systematic review: the hepatotoxicity of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther 2004; 20: 373-80. [PubMed: 15298630](NSAIDs are the most commonly used drugs in the US and account for a large proportion of cases of hepatic injury, but the frequency is quite rare. Among 7 population based studies, hospitalization occurred in 22.4/100,000 patient-years of NSAID use [rate ratio=1.5] and deaths from liver injury occurred in ~1/100,000 patient-years; frequency of injury did not increase with age and was no more common in women than men; in case controlled studies, higher odds ratio for liver injury was found with sulindac, indomethacin, piroxicam and diclofenac; no mention of ketoprofen).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-1101. [PubMed: 16165719](Survey of all cases of fatal drug induced liver injury from Swedish Adverse Drug Reporting system from 1966-2002; among 103 cases, none were attributed to ketoprofen).
- Lapeyre-Mestre M, de Castro AM, Bareille MP, Garcia del Pozo J, Requejo AA, Arias LM, Montastruc J-L, et al. Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. Fundam Clin Pharmacol 2006; 20: 391-5. [PubMed: 16867024](Analysis of reports of liver injury from NSAIDs from France and Spain from 1982-2001; ketoprofen listed as associated with 4 of 2114 [0.2%] hepatic reactions from Spain and 429 of 27,372 [1.5%] from France; details of injury not given).
- Arellano FM, Yood MU, Wentworth CE, Oliveria SA, Rivero E, Verman A, Rothman K. Use of cyclo-oxygenase 2 inhibitors (COX-2) and prescription non-steroidal anti-inflammatory drugs (NSAIDS) in UK and USA populations Implications for COX-2 cardiovascular profile. Pharmacoepidemiol Drug Saf 2006; 15: 861-72. [PubMed: 17086563](Surveys from the UK and USA indicate that ibuprofen, naproxen and diclofenac were the most commonly used NSAIDs; ketoprofen not in top 10 agents used).
- Zabala S, Calpe MJ, Pérez G, Lerín FJ, Mouronval L. Neutropenia, thrombocytopenia and hepatic injury associated with dexketoprofen trometamol therapy in a previously healthy 35-year-old woman. J Clin Pharm Ther 2008; 33: 79-81. [PubMed: 18211621](35 year old woman developed fever 5 days after starting dexketoprofen [active enantiomer of racemic ketoprofen, available in UK] and stopped at ten days [bilirubin 0.5 mg/dL, ALT 216 U/L, Alk P 105 U/L], with low platelet [94,000/μL] and white cell counts [1,600/μL] and resolution within 2 weeks of stopping).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, NSAIDs were implicated as a sole agent in 8 cases [4 diclofenac, 2 celecoxib, 1 meloxicam and 1 oxaprozin] and as one of several agents in 3 cases [1 diclofenac, 1 celecoxib, 1 ibuprofen]; none were attributed to ketoprofen).
- Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol 2010; 16: 5651-61. [PMC free article: PMC2997980] [PubMed: 21128314](Review of estimated frequency of drug induced liver injury due to NSAIDs from large published epidemiological studies; no discussion of ketoprofen).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 7 to NSAIDs, including 4 to bromfenac, 2 diclofenac and 1 etodolac, but none to ketoprofen, ibuprofen or naproxen).
- Famularo G, Gasbarrone L, Minisola G. Probable ketoprofen-associated nonalcoholic fatty liver disease and steatohepatitis. Ann Pharmacother 2011; 45: 423. [PubMed: 21364038](45 year old woman developed abdominal pain and fever 5 days after starting ketoprofen [bilirubin 2.8 mg/dL, ALT 612 U/L, Alk P 810 U/L, without eosinophilia], resolving within 1 week of stopping; she had tolerated diclofenac and ketorolac in the past).
- Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, Jové J, Gatta A, et al. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case-population SALT study. Drug Saf 2013; 36: 135-44. [PMC free article: PMC3568201] [PubMed: 23325533](Among 600 patients undergoing liver transplantation for acute liver failure at 52 European liver transplant centers between 2005 and 2007, 301 were considered idiopathic and had received a medication within 30 days of onset, including acetaminophen in 192 and NSAIDs in 40, including ketoprofen in 3 for a rate of 1.55 per million-treatment years).
- Lapeyre-Mestre M, Grolleau S, Montastruc JL; Association Française des Centres Régionaux de Pharmacovigilance (CRPV). Adverse drug reactions associated with the use of NSAIDs: a case/noncase analysis of spontaneous reports from the French pharmacovigilance database 2002-2006. Fundam Clin Pharmacol 2013; 27: 223-30. [PubMed: 21929527](Analysis of serious adverse events reporting to a French pharmacovigilance database found highest cumulative rates for liver related reports for nimesulide [0.15 per million defined daily doses], followed by diclofenac [0.09], ketoprofen [0.09], piroxicam [0.06], naproxen [0.04] and meloxicam [0.03], being significant in case/noncase analyses for nimesulide, diclofenac and piroxicam only).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 6 attributed to diclofenac (ranking 2nd), but none due to ketoprofen).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common class of implicated agents being NSAIDS [n=62, 32%], but specific agents were nimesulide [n=53], piroxicam [5], diclofenac [2], gold salts [1], and naproxen [1]; ketoprofen was not listed]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 28 were attributed to NSAIDs, but none to ketoprofen [Schmeltzer 2016]).
- Schmeltzer PA, Kosinski AS, Kleiner DE, Hoofnagle JH, Stolz A, Fontana RJ, Russo MW; Drug-Induced Liver Injury Network (DILIN). Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int 2016; 36: 603-9. [PMC free article: PMC5035108] [PubMed: 26601797](Among 1221 cases of drug induced liver injury enrolled in a prospective, US database between 2004 and 2014, 30 cases [2.5%] were attributed to NSAIDs, most commonly diclofenac; none were attributed to ketoprofen).
- Donati M, Conforti A, Lenti MC, Capuano A, Bortolami O, Motola D, Moretti U, et al; DILI-IT Study Group. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy. Br J Clin Pharmacol 2016; 82: 238-48. [PMC free article: PMC4917796] [PubMed: 26991794](Among 179 cases of acute liver injury and 1770 controls admitted to 9 Italian hospitals between 2010 and 2014, NSAIDs used more frequently in cases compared to controls included nimesulide [17% vs 10%: odds ratio 1.88] and ibuprofen [14% vs 10%: odds ratio 1.59] whereas ketoprofen was being taken at a similar rate by cases [11%] as controls [10%]).
- Zoubek ME, González-Jimenez A, Medina-Cáliz I, Robles-Díaz M, Hernandez N, Romero-Gómez M, Bessone F, et al. High Prevalence of ibuprofen drug-induced Liver injury in Spanish and Latin-American registries. Clin Gastroenterol Hepatol 2018; 16: 292-294. [PubMed: 28782674](Analysis of a Spanish and Latin-American registries identified 73 cases of NSAID induced liver injury, the most common agents being nimesulide [38%], diclofenac [34%] and ibuprofen [17%]; ketoprofen was not mentioned).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Fenoprofen.[LiverTox: Clinical and Researc...]Review Fenoprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Flurbiprofen.[LiverTox: Clinical and Researc...]Review Flurbiprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Tolmetin.[LiverTox: Clinical and Researc...]Review Tolmetin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Nimesulide.[LiverTox: Clinical and Researc...]Review Nimesulide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Diclofenac.[LiverTox: Clinical and Researc...]Review Diclofenac.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Ketoprofen - LiverToxKetoprofen - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...