NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsDALBAVANCIN, ORITAVANCIN, TELAVANCIN
OVERVIEW
Introduction
The glycopeptide antibiotics are semisynthetic macromolecules that are structurally related to vancomycin and have antibacterial activity against several gram positive organisms including methicillin resistant Staphylococcus aureus (MRSA). Three lipoglycopeptide antibiotics are available for use in the United States: dalbavancin, oritavancin and telavancin. All three agents have been associated with transient serum enzyme elevations during therapy, but they have yet to be linked convincingly to cases of clinically apparent acute liver injury.
Background
The glycopeptide antibiotics are a class of antimicrobial agents that share a similar, macromolecular structure and basic mechanism of action. The lipoglycopeptides are largely synthetic derivatives of vancomycin modified to have greater potency or better pharmacokinetics and tolerance. Like vancomycin, these agents have potent antibacterial activity against several Streptococcus and Enterococcus species as well as Staphylococcus aureus, including strains that are methicillin-resistant (MRSA). The agents are macromolecular glycopeptides that act by binding to nascent bacterial cell wall peptidoglycans, thus interfering with cell wall synthesis. The lipoglycopeptides are not absorbed orally and have a prolonged half-life when given intravenously which allows for once daily and, in some cases, once weekly administration. Vancomycin is a similar glycopeptide, but its indications, wide scale use and record of safety are greater than the more recently approved lipoglycopeptide derivatives. Vancomycin is discussed in a separate document in LiverTox. Three lipoglycopeptide antibiotics have been approved for use in the United States and are discussed together: telavancin (2009), dalbavancin (2014) and oritavancin (2014).
Telavancin (tel" a van' sin) is a macromolecular semisynthetic lipoglycopeptide with potent antimicrobial activity against several gram positive organisms including Staphylococcus aureus. The activity of telavancin against methicillin-resistant S. aureus (MRSA) infections has made it a valuable agent in the management of community and nosocomial acquired severe MRSA related infections. Telavancin was approved for use in the United States in 2009 for severe infections of the skin and skin structures due to susceptible organisms, and the indications were subsequently broadened to therapy of severe nosocomial pneumonia. Telavancin is available in solution in single use vials of 250 and 750 mg under the commercial name Vibativ. The recommended dose in adults is 10 mg/kg intravenously given over a 60 minute period once daily for 7 to 21 days. Side effects include infusion reactions, nausea, diarrhea, taste disturbance and foamy urine. Telavancin can also cause renal dysfunction and prolongation of the QTc interval. Rare side effects include hypersensitivity reactions, renal failure and Clostridium difficile (pseudomembranous) colitis.
Dalbavancin (dal" ba van' sin) is a macromolecular semisynthetic lipoglycopeptide with potent spectrum of antimicrobial activity against several gram positive organisms including Staphylococcus aureus. The activity of dalbavancin against MRSA infections has made it a valuable agent in the management of community and nosocomial acquired severe MRSA related infections. Dalbavancin was approved for use in the United States in 2014 for severe skin and skin structure infections due to susceptible organisms including MRSA. It is available as a powder for suspension in vials of 500 mg under the commercial name Dalvance. The recommended dose in adults is a single infusion of 1000 mg given intravenously over a 30 minute period, followed one week later by 500 mg intravenously. It can also be given as a single 1500 mg intravenous infusion. Side effects can include infusion reactions (“red man syndrome”), headache, nausea, diarrhea, rash and pruritus. Rare side effects include hypersensitivity reactions and Clostridium difficile (pseudomembranous) colitis.
Oritavancin (or it" a van' sin) is a macromolecular semisynthetic lipoglycopeptide with potent antimicrobial activity against several gram positive organisms including Staphylococcus aureus. The activity of oritavancin against MRSA infections has made it a valuable agent in the management of community and nosocomial acquired severe MRSA-related infections. Oritavancin was approved for use in the United States in 2014 for severe skin and skin structure infections due to susceptible organisms including MRSA. It is available as a sterile powder for suspension in single use vials of 400 mg under the commercial name Orbactiv. The recommended dose in adults is a single infusion of 1200 mg intravenously over a 3 hour period. Side effects include infusion reactions, headache, nausea, diarrhea, skin rash and pruritus. Rare side effects include hypersensitivity reactions and Clostridium difficile (pseudomembranous) colitis.
Hepatotoxicity
In prelicensure controlled trials, serum ALT elevations during therapy with dalbvancin, oritavancin or telavancin were common, occurring in up to 25% of patients. Serum aminotransferase elevations above three times the upper limit of normal, however, were uncommon, occurring in 0.8% to 6% of patients receiving dalbavancin, oritavancin or telavancin. Furthermore, these rates of liver test abnormalities were not very different from those in comparator arms. The ALT elevations during glycopeptide antibiotic therapy were in general transient, asymptomatic and only mild-to-moderate in severity, rarely leading to dose modifications or early discontinuations. There were no reports of clinically apparent liver injury with jaundice in the registration trials of these agents. Since their approval and more wide scale use, there have been no published reports of liver injury attributed to glycopeptide antibiotics, although hypersensitivity reactions have been reported which can sometimes be associated with a mild-to-moderate degree of liver injury. Regardless, these three agents are relatively new, have not been widely used and when used, are given for a relatively short period of time and none have been linked to serious cases of liver injury.
Likelihood score: E (unlikely causes of clinically apparent liver injury).
Mechanism of Injury
The mechanism of the serum aminotransferase elevations and mild liver injury that can occur during intravenous therapy with glycopeptide antibiotics is not known. All three of the currently available lipoglycopeptides have only minor hepatic metabolism and they have no or only weak effects on the activity of the microsomal cytochrome P450 enzymes.
Outcome and Management
The serum enzyme elevations that occur during dalbavancin, oritavancin and telavancin therapy are generally mild and self-limited and rarely require dose modification. There have been no reports of acute liver failure, chronic hepatitis or vanishing bile duct syndrome associated with the glycopeptide antibiotics. There is likely to be some degree of cross sensitivity to the acute hypersensitivity reactions that can occur with infusions of the different glycopeptides.
Drug Class: Antiinfective Agents
Other Drugs in the Subclass, Glycopeptide Antibiotics: Vancomycin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Dalbavancin – Dalvance®
Oritavancin – Orbactiv®
Telavancin – Vibativ®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Dalbavancin | 171500-79-1 | C88-H100-Cl2-N10-O28 |
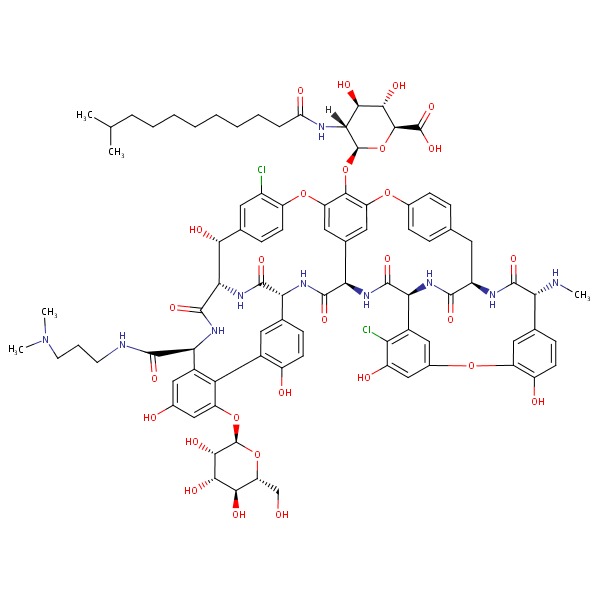 |
| Oritavancin | 171099-57-3 | C80-H106-Cl2-N11-O27-P |
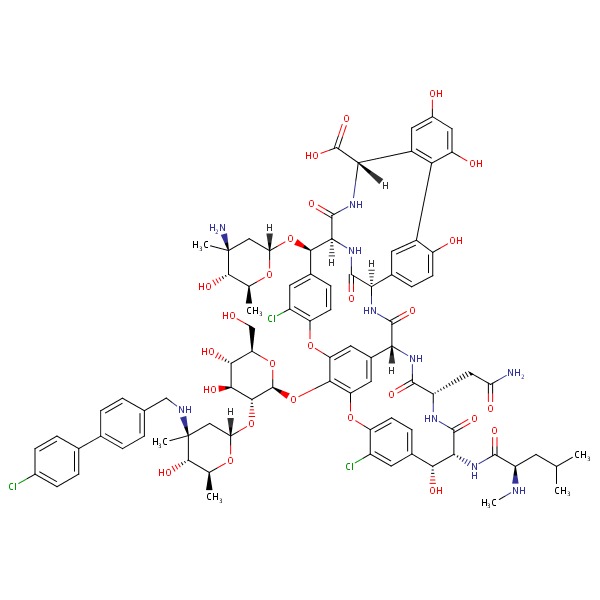 |
| Telavancin | 372151-71-8 | C80-H106-Cl2-N11-O27-P |
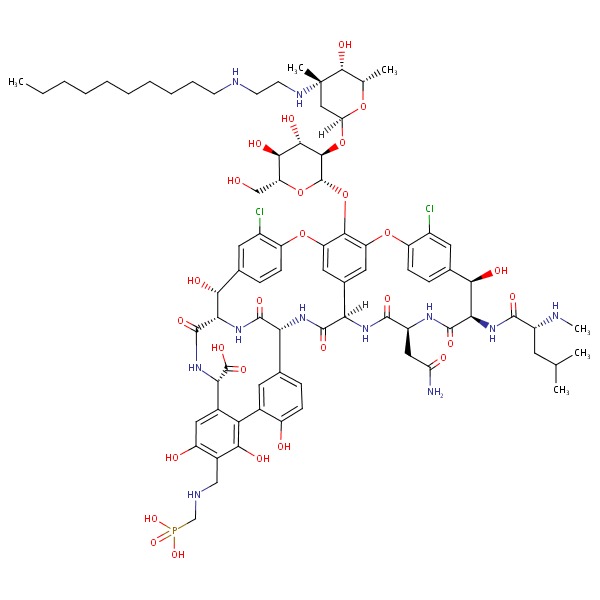 |
| Vancomycin | 1404-90-6 | C66-H75-C12-N9-O24 |
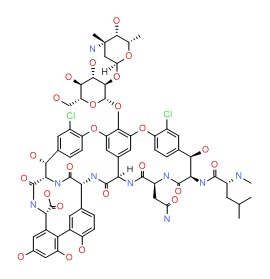 |
ANNOTATED BIBLIOGRAPHY
References updated: 12 November 2017
- Moseley RH. Vancomycin. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced Liver Disease. 3rd edition. Amsterdam: Elsevier, 2013, p. 469.(Expert review of antibiotic induced liver injury discusses vancomycin, but not the other glycopeptide antibiotics).
- MacDougall C, Chambers HF. Protein synthesis inhibitors and miscellaneous antibacterial agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1521-47.(Textbook of pharmacology and therapeutics).
- Billeter M, Zervos MJ, Chen AY, Dalovisio JR, Kurukularatne C. Dalbavancin: a novel once-weekly lipoglycopeptide antibiotic. Clin Infect Dis 2008; 46: 577-83. [PubMed: 18199045](Review of the mechanism of action, efficacy and safety of dalbavancin mentions that it has been used in more than 1000 patients and “there is no known evidence of renal or hepatic toxicity”).
- Stryjewski ME, Graham DR, Wilson SE, O'Riordan W, Young D, Lentnek A, Ross DP, et al.; Assessment of Telavancin in Complicated Skin and Skin-Structure Infections Study. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin Infect Dis 2008; 46: 1683-93. [PubMed: 18444791](Among 1867 patients with severe bacterial skin or skin structure infections treated with telavancin or vancomycin, clinical cure rates were similar [88% vs 87%] and common side effects included taste disturbance [33% vs 7%], nausea [27% vs 15%], headache [14% vs 13%], foamy urine [13% vs 3%], renal dysfunction [3% vs 1%], ALT elevations above 3 times ULN [2% vs 4%] and increase in QTc [1.2% vs 0.6%]).
- Telavancin (Vibativ) for gram-positive skin infections. Med Lett Drugs Ther 2010; 52 (1329): 1-2. [PubMed: 20208468](Concise summary of the mechanism of action, clinical efficacy, side effects and costs of telavancin shortly after its approval in the US, mentions common side effects of nausea, diarrhea, renal dysfunction and taste disturbance and rare side effects of renal failure [~3%] and prolongation of the QTc interval).
- Rubinstein E, Lalani T, Corey GR, Kanafani ZA, Nannini EC, Rocha MG, Rahav G, et al.; ATTAIN Study Group. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis 2011; 52: 31-40. [PMC free article: PMC3060890] [PubMed: 21148517](Among 1503 patients with severe hospital acquired pneumonia treated with telavancin [10 mg/kg/day] or vancomycin [1 g every 12 hours] for 7-21 days, cure rates were similar [59% vs 59.5%], creatinine elevations occurred in 16% vs 10% and ALT elevations above 3 times ULN in 6% vs 8%).
- Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 2014; 370: 2169-79. [PubMed: 24897082](Among 1312 patients with acute skin and skin structure infections treated with dalbavancin [day 1 and 8] or vancomycin [days 1-3] with an option to receive oral linezolid [days 4-14], clinical response rates were similar [80% vs 80%] and common side effects included nausea, diarrhea and pruritus; ALT elevations occurred in 23% of both groups but were usually mild, being above 3 times ULN occurred in 1.4% vs 0.2% of patients).
- Two new drugs for skin and skin structure infections. Med Lett Drugs Ther 2014; 56 (1449): 73-5. [PubMed: 25118799](Concise review of the mechanism of action, efficacy, safety and cost of dalbavancin therapy shortly after its approval in the US mentions side effects of nausea, diarrhea, headache and infusion reactions [“red man syndrome”]; no mention of ALT elevations or hepatotoxicity).
- Corey GR, Kabler H, Mehra P, Gupta S, Overcash JS, Porwal A, Giordano P, et al.; SOLO I Investigators. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med 2014 Jun 5; 370 (23): 2180-90. [PubMed: 24897083](Among 954 patients with severe bacterial skin and skin structure infections treated with oritavancin [single 1200 mg dose] or vancomycin [twice daily for 7 to 10 days], clinical cure rates were similar [80% vs 80%] as were adverse event rates; abnormal liver tests occurred in 2.3% of oritavancin vs 1.0% of vancomycin treated subjects, but none developed clinically apparent liver injury with jaundice).
- Barriere SL. The ATTAIN trials: efficacy and safety of telavancin compared with vancomycin for the treatment of hospital-acquired and ventilator-associated bacterial pneumonia. Future Microbiol 2014; 9: 281-9. [PubMed: 24450506](Among 1532 patients with severe hospital acquired pneumonia treated with telavancin vs vancomycin, cure rates were similar [58% vs 59%] but all-cause mortality was higher with telavancin [39% vs 30%], while side effect rates were similar; no mention of ALT elevations or hepatotoxicity).
- Oritavancin (Orbactiv) for skin and skin structure infections. Med Lett Drugs Ther 2015; 57 (1459): 3-5. [PubMed: 25555072](Concise summary of the mechanism of action, clinical efficacy, safety and costs of oritavancin shortly after its approval in the US for severe skin infections, mentions common side effects of nausea [10%], headache [7%] and diarrhea [4%], and that it does not have the renal and disturbed taste side effects of telavancin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 3 cases were attributed to vancomycin, but none to the other glycopeptide antibiotics).
- Dunne MW, Puttagunta S, Giordano P, Krievins D, Zelasky M, Baldassarre J. A randomized clinical trial of single-dose versus weekly dalbavancin for treatment of acute bacterial skin and skin structure infection. Clin Infect Dis 2016; 62: 545-51. [PMC free article: PMC4741365] [PubMed: 26611777](Among 698 patients with skin or skin structure bacterial infections treated with 1500 mg of dalbavancin as either a single or as two infusions one week apart, the clinical response rates were similar [81% vs 84%] and adverse events rates were the same [20% vs 20%]; no mention of ALT elevations or hepatotoxicity).
- Dunne MW, Talbot GH, Boucher HW, Wilcox M, Puttagunta S. Safety of dalbavancin in the treatment of skin and skin structure infections: a pooled analysis of randomized, comparative studies. Drug Saf 2016; 39: 147-57. [PMC free article: PMC4735234] [PubMed: 26715497](Among 1778 patients with skin or skin structure bacterial infections treated with dalbavancin and 1224 who received comparator agents in phase 2 and 3 studies, common side effects of dalbavancin included nausea [5.5% vs 6.4%], headache [4.7% vs 4.8%], diarrhea [4.4% vs 5.9%], rash [2% vs 1.8%] and pruritus [1.8% vs 2.9%]; ALT abnormalities of any degree occurred in 24% vs 26% of subjects and were above 3 times ULN in 2.6% vs 2.6%; no patient developed clinically apparent liver injury with jaundice).
- Bassetti M, Righi E. Safety profiles of old and new antimicrobials for the treatment of MRSA infections. Expert Opin Drug Saf 2016; 15 (4): 467-81. [PubMed: 26764972](Summary and overview of the safety of recently developed drugs for MRSA infections including telavancin, dalbavancin and oritavancin mentions transient ALT elevations during therapy, but no mention of clinically apparent hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review A comparative review of the lipoglycopeptides: oritavancin, dalbavancin, and telavancin.[Pharmacotherapy. 2010]Review A comparative review of the lipoglycopeptides: oritavancin, dalbavancin, and telavancin.Guskey MT, Tsuji BT. Pharmacotherapy. 2010 Jan; 30(1):80-94.
- Review New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin.[Drugs. 2010]Review New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin.Zhanel GG, Calic D, Schweizer F, Zelenitsky S, Adam H, Lagacé-Wiens PR, Rubinstein E, Gin AS, Hoban DJ, Karlowsky JA. Drugs. 2010 May 7; 70(7):859-86.
- Review Beyond Vancomycin: The Tail of the Lipoglycopeptides.[Clin Ther. 2015]Review Beyond Vancomycin: The Tail of the Lipoglycopeptides.Klinker KP, Borgert SJ. Clin Ther. 2015 Dec 1; 37(12):2619-36. Epub 2015 Dec 3.
- Review Recent Advances in the Development of Semisynthetic Glycopeptide Antibiotics: 2014-2022.[ACS Infect Dis. 2022]Review Recent Advances in the Development of Semisynthetic Glycopeptide Antibiotics: 2014-2022.van Groesen E, Innocenti P, Martin NI. ACS Infect Dis. 2022 Aug 12; 8(8):1381-1407. Epub 2022 Jul 27.
- Review Antibacterial activity of recently approved antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) strains: A systematic review and meta-analysis.[Ann Clin Microbiol Antimicrob....]Review Antibacterial activity of recently approved antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) strains: A systematic review and meta-analysis.Liu F, Rajabi S, Shi C, Afifirad G, Omidi N, Kouhsari E, Khoshnood S, Azizian K. Ann Clin Microbiol Antimicrob. 2022 Aug 17; 21(1):37. Epub 2022 Aug 17.
- Glycopeptide Antibiotics - LiverToxGlycopeptide Antibiotics - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...