NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Glucosamine is a popular nutritional supplement and natural component of cartilage that is frequently combined with chondroitin sulfate and used for osteoarthritis and nonspecific joint pain. Glucosamine has been implicated in isolated case reports in causing clinically apparent liver injury, but the role of glucosamine as opposed to other herbal components or contaminants has not been shown, and liver injury due to glucosamine or chondroitin must be very rare if it occurs at all.
Background
Glucosamine (gloo kose' a meen) is a natural component of cartilage that is a widely used as an over-the-counter nutritional supplement purported to decrease the pain and cartilage loss of osteoarthritis. Glucosamine is commonly taken in combination with chondroitin (kon droe' i tin), which is a glycosaminoglycan that is also present in cartilage. Glucosamine is an amino sugar and a prominent molecule in the biochemical pathways of synthesis of glycosylated proteins and lipids. It is also a major component of keratin sulfate and hyaluronic acid which are present in articular cartilage and synovial fluid. Both glucosamine and chondroitin are reduced in osteoarthritis. Glucosamine is commercially available alone and in combination with chondroitin and widely used for osteoarthritis and arthritic pain. Controlled trials of glucosamine with and without chondroitin have yielded conflicting results. In the largest US study of glucosamine for early osteoarthritis, glucosamine alone and the combination of glucosamine and chondroitin were no more beneficial than placebo in alleviating joint pain or preventing progression of cartilage damage. Glucosamine is typically taken in doses of 500 mg three times daily and chondroitin sulfate in doses of 200 to 400 mg three times daily. Side effects are uncommon and mild and may include abdominal discomfort, nausea, fatigue and headache; but in placebo controlled trials, side effects during glucosamine therapy were no more frequent than with receipt of placebo.
Hepatotoxicity
In controlled trials, glucosamine and its combination with chondroitin have not been linked to serum enzyme elevations or to instances of clinically apparent liver injury. In addition, cases of clinically apparent liver injury have not been reported from prospective trials. Recently, several cases reports and small series of clinically apprent liver injury attributed to glucosamine (with or without chondroitin) have been published, but the relationship of glucosamine itself as opposed to other herbals in the implicated products or to potential contaminants, remains unclear and several cases were considered only "possibly" related to glucosamine. The time to onset is usually 1 to 4 weeks after starting the preparation and the pattern of injury is typically hepatocellular or mixed. At least one instance of acute liver failure has been reported. Immunoallergic features (rash, fever, eosinophilia) can occur, but are usually not prominent. Most patients were reported to recover within 4 to 8 weeks of stopping. There have not been instances of rechallenge with glucosamine, and the purity and concentration of glucosamine in the products used have not been reported.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which glucosamine or chondroitin might cause liver injury is unclear. Glucosamine is a simple amino sugar and chondroitin a glycosaminoglycan. Both are natural products found in cartillage in humans and mammals. The glucosamine in commercially available dietary supplements is usually prepared from the exoskeletons of shellfish or from fermentation of grain. The concentration, purity and freedom from contaminants in commercially available preparations of glucosamine and chondroitin is not always clear.
Outcome and Management
The severity of the liver injury in reports of glucosamine hepatotoxicity has varied from mild, asymptomatic elevations in serum enzymes to clinically apparent hepatitis that can be severe and even fatal. There is no information on management or the role for corticosteroids or other interventions except for prompt discontinuation of the suspected agent.
Drug Class: Herbal and Dietary Supplements
CASE REPORT
Case 1. Acute liver injury attributed to a glucosamine containing dietary and herbal supplement.
[Modified from: Ossendza RA, Grandval P, Chinoune F, Rocher F, Chapel F, Bernardini D. [Acute cholestatic hepatitis due to glucosamine forte]. Gastroenterol Clin Biol 2007; 31: 449-50. French.]
A 52 year old man developed weakness followed by jaundice and generalized itching arising 3 weeks after starting capsules of Glucosamine forte. He had no previous history of liver disease or drug allergies and denied alcohol abuse and risk factors for viral hepatitis. He had no other major medical illnesses and was taking glucosamine for low back pain. On presentation, physical examination revealed jaundice, but no fever or rash. Laboratory testing showed a total bilirubin of 10.9 mg/dL, ALT 263 U/L, AST 63 U/L, GGT 240 U/L and alkaline phosphatase 714 U/L (Table). He tested negative for markers of hepatitis A, B, C and E as well as EBV, cytomegalovirus and herpes simplex. Tests for autoantibodies were negative. Abdominal ultrasound and CT scans were normal with no evidence of biliary obstruction. A liver biopsy showed canalicular cholestasis, hepatocellular necrosis and lobular inflammation with one microgranuloma, which overall was considered compatible with drug induced liver injury. Glucosamine had been stopped a week before clinical presentation and his symptoms improved rapidly. Serum bilirubin rose to 13.9 and then gradually began to fall. Liver tests fell into the near normal range within 8 weeks of stopping the supplement and were completely normal when he was seen 4 months later.
Key Points
| Medication: | Glucosamine |
|---|---|
| Pattern: | Mixed (R=2.5) |
| Severity: | 3+ (jaundice and hospitalized) |
| Latency: | 3 weeks to symptoms, 4 weeks to jaundice |
| Recovery: | 8 weeks |
| Other medications: | None |
Laboratory Values
- *
Some values estimated from Figure 1.
Comment
This was a typical case of cholestatic-mixed hepatitis due to a medication, but in this instance the only drug being taken was a commercial preparation of glucosamine. Typical of cholestatic hepatitis was the presentation with pruritus and the somewhat delayed recovery. While the dietary supplement appears to be fairly convincingly implicated as the cause of the liver injury, what is uncertain is whether glucosamine per se versus a possible contaminant was responsible. Glucosamine is a simple amino sugar and is used by millions of people. Reports of hepatic injury from glucosamine are few (less than a dozen in the published literature) and always limited by the lack of proof of purity of the dietary supplement used.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Glucosamine – Generic
Chondroitin sulfate – Generic
DRUG CLASS
Herbal and Dietary Supplements
SUMMARY INFORMATION
(Glucosamine Hydrochloride) Fact Sheet at MedlinePlus, NLM
(Glucosamine Sulfate) Fact Sheet at MedlinePlus, NLM
(Chondroitin Sulfate) Fact Sheet at MedlinePlus, NLM
Fact Sheet at National Center for Complementary and Integrative Health, NIH
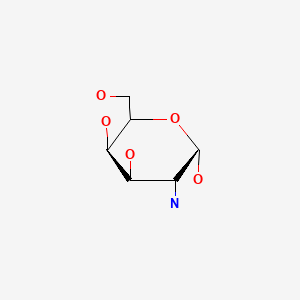
CHEMICAL FORMULAS AND STRUCTURES
ANNOTATED BIBLIOGRAPHY
References updated: 12 March 2018
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott,1999: pp. 731-4.(Expert review of hepatotoxicity published in 1999; glucosamine and chondroitin are not discussed).
- Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbs and dietary supplements. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 631-58.(Review of hepatotoxicity of herbal and dietary supplements [HDS]; glucosamine and chondroitin are not discussed).
- Glucosamine. In, PDR for Herbal Medicines. 4th ed. Montvale, New Jersey: Thomson Healthcare Inc. 2007: pp. 967-71.(Compilation of short monographs on herbal medications and dietary supplements).
- Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, Giacovelli G, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–6. [PubMed: 11214126](Controlled trial from Belgium of 3 years of glucosamine sulphate vs placebo in 212 patients with osteoarthritis of the knees; found no differences in side effects and no serious adverse events attributable to glucosamine; no mention of hepatotoxicity or results of liver tests).
- Pavelká K, Gatterová J, Olejarová M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162:2113–23. [PubMed: 12374520](Controlled trial of from Italy of glucosamine sulphate vs placebo in 202 patients with knee osteoarthritis; there were no significant differences in frequency of adverse events between the two groups or in safety laboratory results; hepatotoxicity and ALT levels were not mentioned).
- De Smet PAGM. Herbal remedies. N Engl J Med. 2002;347:2046–56. [PubMed: 12490687](Review of status and difficulties of herbal medications including lack of standardization, federal regulation, contamination, safety, hepatotoxicity and drug-herb interactions; specific discussion of 4 herbs with therapeutic promise: ginkgo, hawthorn, saw palmetto and St. John’s wort).
- Schiano TD. Hepatotoxicity and complementary and alternative medicines. Clin Liver Dis. 2003;7:453–73. [PubMed: 12879994](Comprehensive review of herbal associated hepatotoxicity; glucosamine is not listed as causing hepatotoxicity).
- Ossendza RA, Grandval P, Chinoune F, Rocher F, Chapel F, Bernardini D. Gastroenterol Clin Biol. 2007;31:449–50. [Acute cholestatic hepatitis due to glucosamine forte] French. [PubMed: 17483788](52 year old man developed jaundice and pruritus 1 week after stopping 3 week course of glucosamine for low back pain [bilirubin 10.9 mg/dL, ALT 263 U/L, Alk P 714 U/L, eosinophils 1140/uL], with biopsy showing cholestatic hepatitis and a granuloma, episode resolving within 2 months of stopping).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, including 7 [5%] for herbal medications, none were attributed to glucosamine or chondroitin).
- Rozendaal RM, Koes BW, van Osch GJ, Uitterlinden EJ, Garling EH, Willemsen SP, Ginai AZ, et al. Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med. 2008;148:268–77. [PubMed: 18283204](Controlled trial from the Netherlands of 2 years of glucosamine sulfate vs placebo in 222 patients with hip osteoarthritis; there was no significant differences in adverse events between the two groups and no serious adverse events related to the liver).
- García-Cortés M, Borraz Y, Lucena MI, Peláez G, Salmerón J, Diago M, Martínez-Sierra MC, et al. Rev Esp Enferm Dig. 2008;100:688–95. [Liver injury induced by “natural remedies”: an analysis of cases submitted to the Spanish Liver Toxicity Registry] Spanish. [PubMed: 19159172](Among 521 cases of drug induced liver injury submitted to Spanish registry, 13 [2%] were due to herbals but none were attributed to glucosamine or chondroitin).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 9% of cases were attributed to herbal medications or dietary supplements, but none of the cases were linked to glucosamine or chondroitin).
- Smith A, Dillon J. Acute liver injury associated with the use of herbal preparations containing glucosamine: three case studies. BMJ Case Rep 2009; 2009. [PMC free article: PMC3028037] [PubMed: 21887162](Three patients developed liver injury after taking glucosamine: 2 women and 1 man, ages 55-64 years, developed liver injury 5 days, 4 weeks and 6 months after starting the product [bilirubin 24.5, 11.6 mg/dL and normal; ALT 1461, 1130 and 175 U/L, Alk P 141, 198 and 187 U/L], one patient died of acute liver failure, a second developed cirrhosis and a third resolved without symptoms or jaundice; details of products not provided, but some included other potentially hepatotoxic herbals).
- Wilkens P, Scheel IB, Grundnes O, Hellum C, Storheim K. Effect of glucosamine on pain-related disability in patients with chronic low back pain and degenerative lumbar osteoarthritis: a randomized controlled trial. JAMA. 2010;304:45–52. [PubMed: 20606148](Controlled trial from Norway of 6 months of glucosamine vs placebo in 250 patients with back pain and degenerative osteoarthritis; rates of adverse events were similar in the two groups: hepatotoxicity and ALT elevations were not mentioned).
- Sawitzke AD, Shi H, Finco MF, Dunlop DD, Harris CL, Singer NG, Bradley JD, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459–64. [PMC free article: PMC3086604] [PubMed: 20525840](Controlled trial from US of 24 months of glucosamine, chondroitin, their combination, or celecoxib vs placebo in 662 patients with knee osteoarthritis; rates of adverse events were similar across treatment groups and no liver related serious adverse event occurred).
- Miller KL, Clegg DO. Glucosamine and chondroitin sulfate. Rheum Dis Clin North Am. 2011;37:103–18. [PubMed: 21220090](Review of the structure, pharmacokinetics, possible mechanisms of action, clinical efficacy and safety of glucosamine and chondroitin as therapy for osteoarthritis; other than a possible interaction with warfarin, no serious adverse events have been linked to long term use of glucosamine and chondroitin).
- Linnebur SA, Rapacchietta OC, Vejar M. Hepatotoxicity associated with chinese skullcap contained in Move Free Advanced dietary supplement: two case reports and review of the literature. Pharmacotherapy 2010; 30(7): 750, 258e-262e. [PubMed: 20586134](Abstract only: 2 patients developed liver injury after taking "Move Free Advanced", a dietary supplement which contains glucosamine, chondroitin and also extracts of several herbs including Chinese skullcap, which the authors suggested was the responsible agent).
- Ebrahim V, Albeldawi M, Chiang DJ. Acute liver injury associated with glucosamine dietary supplement. BMJ Case Rep 2012; 2012. [PMC free article: PMC4544092] [PubMed: 23239775](55 year old woman developed jaundice 2 weeks after starting glucosamine [bilirubin 9.1 mg/dL, ALT 1553 U/L, Alk P 303 U/L], resolving within 4 weeks of stopping).
- Cerda C, Bruguera M, Parés A. Hepatotoxicity associated with glucosamine and chondroitin sulfate in patients with chronic liver disease. World J Gastroenterol. 2013;19:5381–4. [PMC free article: PMC3752575] [PubMed: 23983444](Transient elevations in serum ALT levels [from 33 and 53 to 282 and 162 U/L] were found in 2 patients with chronic hepatitis C taking glucosamine, which improved upon stopping [falling to 85 and 37 U/L]).
- von Felden J, Montani M, Kessebohm K, Stickel F. Drug-induced acute liver injury mimicking autoimmune hepatitis after intake of dietary supplements containing glucosamine and chondroitin sulfate. Int J Clin Pharmacol Ther. 2013;51:219–23. [PubMed: 23391366](65 year old man developed severe autoimmune hepatitis while taking glucosamine and chondroitin sulfate [bilirubin 7.7 mg/dL, ALT 2744 U/L, Alk P 199 U/L, ANA negative, IgG 1820 mg/dL], which responded to corticosteroid therapy and was judged as "possibly" due to the supplement).
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2013;37:3–17. [PubMed: 23121117](Systematic review of literature on HDS associated liver injury does not mention glucosamine or chondroitin).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 [16%] due to herbal and dietary supplements, but none were attributed to glucosamine or chondroitin).
- Dağ MS, Aydınlı M, Oztürk ZA, Türkbeyler IH, Koruk I, Savaş MC, Koruk M, et al. Drug- and herb-induced liver injury: a case series from a single center. Turk J Gastroenterol. 2014;25:41–5. [PubMed: 24918129](Between 2008 and 2012, 82 patients with drug or herbal supplement induced liver injury were seen at a single referral center in Turkey, 10 [12%] of which were due to HDS products, including 7 due to Teucrium polium [mountain germander] and 3 to green tea extract, but none to glucosamine or chondroitin).
- Rossi S, Navarro VJ. Herbs and liver injury: a clinical perspective. Clin Gastroenterol Hepatol. 2014;12:1069–76. [PubMed: 23924877](Review of HDS induced liver injury including regulatory problems, difficulties in diagnosis and assessing causality; discussed glucosamine based supplements which are occasionally implicated in causing liver injury, but most were multiingredient products and included herbs).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a prospective database between 2004 and 2012, HDS were implicated in 145 [16%], two of which were attributed to "Move Free" which contains glucosamine and chondroitin, but also other HDS components [Navarro 2014]).
- Zeng C, Wei J, Li H, Wang YL, Xie DX, Yang T, Gao SG, et al. Effectiveness and safety of Glucosamine, chondroitin, the two in combination, or celecoxib in the treatment of osteoarthritis of the knee. Sci Rep. 2015;5:16827. [PMC free article: PMC4649492] [PubMed: 26576862](Among).
- Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G., CS/GS Combined Therapy Study Group. Combined treatment With chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients With Knee Osteoarthritis: A Six-Month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:77–85. [PubMed: 27477804](Among).
- Hochberg MC, Martel-Pelletier J, Monfort J, Möller I, Castillo JR, Arden N, Berenbaum F, et al. MOVES Investigation Group. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2016;75:37–44. [PMC free article: PMC4717399] [PubMed: 25589511](Among).
- García-Cortés M, Robles-Díaz M, Ortega-Alonso A, Medina-Caliz I, Andrade RJ. Hepatotoxicity by Dietary Supplements: A tabular listing and clinical characteristics. Int J Mol Sci. 2016;17:537. [PMC free article: PMC4848993] [PubMed: 27070596](Listing of published cases of liver injury from HDS products does not discuss glucosamine and chondroitin).
- Avigan MI, Mozersky RP, Seeff LB. Scientific and regulatory perspectives in herbal and dietary supplement associated hepatotoxicity in the United States. Int J Mol Sci. 2016;17:331. [PMC free article: PMC4813193] [PubMed: 26950122](Overview of the US regulations regarding herbal and dietary supplements and role of FDA, Department of Agriculture, Federal Trade Commission and Office of Dietary Supplements of the NIH in assessment of safety of HDS products including actions taken against Hydroxycut, Lipokinetix and OxyELITE Pro when reports of liver injury appeared in postmarketing phase).
- Brown AC. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem Toxicol 2017; 107(Pt A): 449-71. [PubMed: 27818322](Summary of the US regulations on safety and efficacy of herbal and dietary supplements).
- Brown AC. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem Toxicol 2017; 107(Pt A): 472-501. [PubMed: 27402097](Description of an online compendium of cases of liver toxicity attributed to HDS products, mentions that glucosamine and chondroitin have been implicated in causing liver injury in four publications [Ossendza 2007, Smith 2009, Ebrahim 2012, Cerda 2013]).
- Wong LL, Lacar L, Roytman M, Orloff SL. Urgent liver transplantation for dietary supplements: an under-recognized problem. Transplant Proc. 2017;49:322–5. [PubMed: 28219592](Among 2048 adult liver transplants recipients enrolled in the Scientific Registry of Transplant Recipients [SRTR] between 2003 and 2015, 625 were done for acute hepatic necrosis due to drug induced liver injury, half being due to acetaminophen and the 4th most frequent cause [n=21] being HDS products; does not mention glucosamine or chondroitin).
- de Boer YS, Sherker AH. Herbal and dietary supplement-induced liver injury. Clin Liver Dis. 2017;21:135–49. [PMC free article: PMC5117680] [PubMed: 27842768](Review of the frequency, clinical features, patterns of injury and outcomes of HDS hepatotoxicity with specific mention of anabolic steroids, black cohosh, germander, green tea, pyrrolizidine alkaloids and proprietary multiingredint nutrition supplements [MINS] including "Move Free Advanced" which contains glucosamine and chrondroitin, but also skullcap).
- Vega M, Verma M, Beswick D, Bey S, Hossack J, Merriman N, Shah A, et al. Drug Induced Liver Injury Network (DILIN). The incidence of drug- and herbal and dietary supplement-induced liver injury: preliminary findings from gastroenterologist-based surveillance in the population of the State of Delaware. Drug Saf. 2017;40:783–7. [PMC free article: PMC5699929] [PubMed: 28555362](A prospective, population based registry of cases of drug-induced liver injury occurring in Delaware during 2014, identified 20 cases [2.7 per 100,000] overall, including 6 due to HDS products, all of which were proprietary multiingredient products, none specifically mentioning glucosamine or chondroitin).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- The combined therapy with chondroitin sulfate plus glucosamine sulfate or chondroitin sulfate plus glucosamine hydrochloride does not improve joint damage in an experimental model of knee osteoarthritis in rabbits.[Eur J Pharmacol. 2017]The combined therapy with chondroitin sulfate plus glucosamine sulfate or chondroitin sulfate plus glucosamine hydrochloride does not improve joint damage in an experimental model of knee osteoarthritis in rabbits.Roman-Blas JA, Mediero A, Tardío L, Portal-Nuñez S, Gratal P, Herrero-Beaumont G, Largo R. Eur J Pharmacol. 2017 Jan 5; 794:8-14. Epub 2016 Nov 12.
- Literature Analysis Regarding the Combination of Substances: Glucosamine + Chondroitin in the Treatment of Osteoarthritis.[Ortop Traumatol Rehabil. 2022]Literature Analysis Regarding the Combination of Substances: Glucosamine + Chondroitin in the Treatment of Osteoarthritis.Marczyński W, Tłustochowicz W, Tomaszewski W, Białecki J. Ortop Traumatol Rehabil. 2022 Dec 31; 24(6):407-416.
- Review Chondroitin for osteoarthritis.[Cochrane Database Syst Rev. 2015]Review Chondroitin for osteoarthritis.Singh JA, Noorbaloochi S, MacDonald R, Maxwell LJ. Cochrane Database Syst Rev. 2015 Jan 28; 1(1):CD005614. Epub 2015 Jan 28.
- Review Inappropriate claims from non-equivalent medications in osteoarthritis: a position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO).[Aging Clin Exp Res. 2018]Review Inappropriate claims from non-equivalent medications in osteoarthritis: a position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO).Bruyère O, Cooper C, Al-Daghri NM, Dennison EM, Rizzoli R, Reginster JY. Aging Clin Exp Res. 2018 Feb; 30(2):111-117. Epub 2017 Nov 24.
- Review The role of glucosamine sulfate and chondroitin sulfates in the treatment of degenerative joint disease.[Altern Med Rev. 1998]Review The role of glucosamine sulfate and chondroitin sulfates in the treatment of degenerative joint disease.Kelly GS. Altern Med Rev. 1998 Feb; 3(1):27-39.
- Glucosamine - LiverToxGlucosamine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...