NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Fosfomycin is an orally available, broad spectrum antibiotic used largely for treatment of uncomplicated urinary tract infections. Fosfomycin is associated with a low rate of transient serum enzyme during therapy and with rare cases of clinically apparent acute liver injury with jaundice.
Background
Fosfomycin (fos' foe mye. sin) is an oral, broad spectrum bactericidal antibiotic that is typically used as a single, large oral dose to treat acute cystitis. Fosfomycin (also known as phosphomycin) is a natural product of Streptomyces fradiae that acts by inactivation of a bacterial enzyme necessary for cell wall synthesis. Fosfomycin has a unique structure (an analogue of phosphoenolpyruvate) and is structurally unrelated to other antibiotics. It has activity against both gram-positive and gram-negative bacteria and is excreted largely unchanged and in its active form in the urine, making it useful for treatment of urinary tract infections. Antibacterial resistance is, however, frequent with prolonged use. Fosfomycin was approved for use in the United States in 1997 and current indications are for uncomplicated urinary tract infections caused by susceptible organisms. Fosfomycin is available under the commercial name Monurol in a sachet of 3 grams and is recommended as a single dose. Intravenous formulations of fosfomycin have been used in combination with carbapenems or aminoglycosides as therapy of multidrug resistant bacterial organisms such as methicillin-resistant Staphyloccocus aureus (MRSA), but it has yet to be approved for this use in the United States. The intravenous formulation is generally given in 1 to 2 gram amounts every 6 hours for 3 to 28 days. Oral fosfomycin is well tolerated; common side effects may include diarrhea, nausea, headache and fungal vaginitis. Rare, but potentially serious adverse events include allergic reactions, anaphylaxis and toxic megacolon.
Hepatotoxicity
Serum aminotransferase elevations occur in a small proportion of patients after a single oral dose of fosfomycin (1-2%), but at rates similar to those with comparator antibiotics. Nevertheless, serum enzyme elevations are mentioned as potential adverse events in the product label for fosfomycin. In addition, a small number of cases of clinically apparent liver injury attributed to fosfomycin have been published. The time to onset has been short, within one week of a single oral dose or within the first week of intravenous therapy, and the pattern of liver enzyme elevations has been mixed or hepatocellular. The injury has typically been mild and self-limited, and no cases of fatal acute liver failure, chronic hepatitis or vanishing bile duct syndrome have been convincingly linked to fosfomycin. The number of cases described has been too few to establish a typical clinical pattern, but immunoallergic features and autoimmune markers appear to be uncommon.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the liver injury due to fosfomycin is unknown, but is likely to be immunologically mediated. Fosfomycin has minimal hepatic metabolism (mostly glucuronidation), and drug-drug interactions with it are not expected.
Outcome and Management
The severity of the liver injury linked to fosfomycin has ranged from transient, asymptomatic serum enzyme elevations to clinically apparent hepatitis. Severe but not fatal instances of acute hepatitis have been reported. In one publication, reexposure to fosfomycin after clinically apparent liver injury led to a rapid recurrence. Fosfomycin has a unique chemical structure unlike other antibiotics, and there is evidence for cross sensitivity to hepatic injury between fosfomycin and other antimicrobial agents.
Drug Class: Antiinfective Agents
CASE REPORT
Case 1. Acute hepatitis attributed to fosfomycin.
[Modified from: Matsumori A, Yoneda S, Kobayashi Y, Takeda K, Andoh M, Yamane Y, Nishimura K, et al. [A case of acute severe hepatitis induced by fosfomycin]. Nihon Shokakibyo Gakkai Zasshi 2005; 102: 1207-11. Japanese. PubMed Citation]
A 30 year old, previously healthy man with an upper respiratory infection developed fever, fatigue, and jaundice a few days after a single, oral dose of fosfomycin. He had no history of liver disease, alcohol abuse or drug allergies. He was not taking other medications except for cold remedies. He had fever, but no rash or lymphadenopathy. Laboratory tests showed a total bilirubin of 6.5 mg/dL (direct 3.5 mg/dL), ALT 558 U/L, AST 442 U/L, alkaline phosphatase 649 U/L, GGT 192 U/L and a prothrombin index of 44% (roughly equivalent to an INR of 1.7). He was admitted for observation and management (Table). Tests for hepatitis A, B and C were negative as were tests for CMV and EBV infection. ANA and AMA were negative and IgG levels were minimally elevated (1714 mg/dL). An abdominal ultrasound and enhanced CT showed evidence of severe liver necrosis with atrophy. Within the next few days, he developed asterixis with a worsening of prothrombin time and hyperammonemia (112 μg/dL). He was treated with plasma exchange given infusions of glucagon and insulin, whereupon he began to improve. A liver biopsy done 6 weeks after presentation showed spotty necrosis, inflammation and regeneration. He was discharged after 42 days in the hospital. All laboratory tests were normal when he was seen in follow up, 4 months after onset.
Key Points
| Medication: | Fosfomycin, single oral dose |
|---|---|
| Pattern: | Mixed (R=~2.8) |
| Severity: | 4+ (jaundice, hospitalization and features of hepatic failure) |
| Latency: | 3 days |
| Recovery: | Within 4 months |
| Other medications: | Over-the-counter cold remedies |
Laboratory Values
| Time After Starting/Stopping | ALT* (U/L) | Prothrombin Index* | Bilirubin* (mg/dL) | Comments |
|---|---|---|---|---|
| 3 days | 558 | 44% | 6.5 | Admission |
| 6 days | 540 | 40% | 6.5 | Plasma exchange |
| 8 days | 480 | 35% | 6.4 | Plasma exchange |
| 11 days | 220 | 30% | 3.5 | Plasma exchange |
| 13 days | 130 | 33% | 3.0 | |
| 16 days | 160 | 37% | 3.8 | |
| 20 days | 150 | 45% | 2.0 | |
| 30 days | 100 | 75% | 1.4 | |
| 42 days | 50 | 80% | 1.2 | Liver biopsy, discharged |
| 4 months | 25 | 95% | 0.8 | |
| Normal | Not Given | >80% | <1.2 |
*Values estimated from Figure 3.
Comment
A young man developed a severe hepatitis a few days after receipt of a single, large oral dose of fosfomycin for a suspected upper respiratory infection. The pattern of liver enzyme elevations was "mixed", but the course resembled acute liver failure. Other causes of liver disease were adequately ruled out, although tests for hepatitis E and a few rare opportunistic viral infections were not done. Evidence of hepatic failure included prolongation of prothrombin time, hyperammonemia, asterixis and CT and ultrasound evidence of severe hepatic necrosis. The few other reported cases of clinically apparent liver injury from fosfomycin have been self-limited, with mild jaundice and a hepatocellular pattern of serum enzyme elevations. Fosfomycin has been used extensively abroad, in Europe and Asia, but has had limited use in the United States. As might be expected, cases of drug induced liver injury from fosfomycin have been reported from Japan, China and Europe, but not the United States. [Help in the translation of this case report was kindly provided by Dr. Saturo Saito.]
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Fosfomycin – Monurol®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
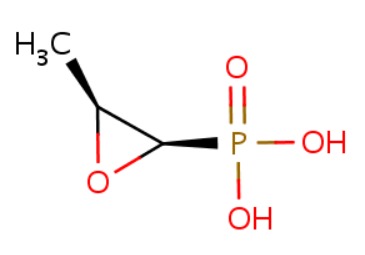
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Fosfomycin | 23155-02-4 | C3-H7-O4-P |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 06 February 2018
- Zimmerman HJ. Hepatic injury from the treatment of infectious and parasitic diseases. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 589-637.(Expert review of hepatotoxicity of antibiotics published in 1999 does not mention fosfomycin).
- Moseley RH. Antibacterial and Antifungal Agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013. p. 463-81.(Review of hepatotoxicity of antibacterial medications; does not discuss fosfomycin).
- de Jong Z, Pontonnier F, Plante P. Single-dose fosfomycin trometamol (Monuril) versus multiple-dose norfloxacin: results of a multicenter study in females with uncomplicated lower urinary tract infections. Urol Int 1991; 46: 344-8. [PubMed: 1926651](Among 63 patients given fosfomycin or norfloxacin for uncomplicated urinary tract infections, efficacy was similar, but side effects were fewer and of shorter duration with fosfomycin; one patient on norfloxacin, but none on fosfomycin had "change in hepatic function").
- Van Pienbroek E, Hermans J, Kaptein AA, Mulder JD. Fosfomycin trometamol in a single dose versus seven days nitrofurantoin in the treatment of acute uncomplicated urinary tract infections in women. Pharm World Sci 1993; 15: 257-62. [PubMed: 8298585](Among 231 women with urinary tract infections managed in general practices in the Netherlands who were treated with a single dose of fosfomycin or 7 days of nitrofurantoin, clinical cure rates were similar and side effects were generally mild and self-limited; no mention of ALT elevations or hepatotoxicity).
- Mayama T, Yokota M, Shimatani I, Ohyagi H. Analysis of oral fosfomycin calcium (Fosmicin) side-effects after marketing. Int J Clin Pharmacol Ther Toxicol 1993; 31: 77-82. [PubMed: 8458680](Analysis of spontaneous reports of adverse events associated with the first six years of clinical use of fosfomycin capsules from 28,238 patients in Japan found 2.9% frequency of gastrointestinal symptoms and 0.2% rate of "disorder of liver and bile duct", but no case associated with jaundice).
- Elhanan G, Tabenkin H, Yahalom R, Raz R. Single-dose fosfomycin trometamol versus 5-day cephalexin regimen for treatment of uncomplicated lower urinary tract infections in women. Antimicrob Agents Chemother 1994; 38: 2612-4. [PMC free article: PMC188250] [PubMed: 7872756](Among 112 women with urinary tract infections treated with either cephalexin or fosfomycin, efficacy was similar in the two groups and there were no significant adverse events attributed to fosfomycin).
- Patel SS, Balfour JA, Bryson HM. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 1997; 53: 637-56. [PubMed: 9098664](Review of the mechanism of action, efficacy and safety of fosfomycin mentions that reported serious adverse events included angioedema, asthma, cholestatic jaundice, hepatic necrosis, toxic megacolon and optic neuritis).
- Fosfomycin for urinary tract infections. Med Lett Drugs Ther 1997; 39 (1005): 66-8. [PubMed: 9255237](Concise review of the activity, efficacy, side effects and costs of fosfomycin for urinary tract infections shortly after its approval for this use in the US; no mention of hepatotoxicity or ALT elevations).
- Minassian MA, Lewis DA, Chattopadhyay D, Bovill B, Duckworth GJ, Williams JD. A comparison between single-dose fosfomycin trometamol (Monuril) and a 5-day course of trimethoprim in the treatment of uncomplicated lower urinary tract infection in women. Int J Antimicrob Agents 1998; 10: 39-47. [PubMed: 9624542](Among 547 women with urinary tract infections treated by general practitioners with either a single oral dose of fosfomycin or a 5 day course of trimethoprim, efficacy was similar; side effects were not discussed).
- Stein GE. Comparison of single-dose fosfomycin and a 7-day course of nitrofurantoin in female patients with uncomplicated urinary tract infection. Clin Ther 1999; 21: 1864-72. [PubMed: 10890258](Among 749 women with urinary tract infections treated with a single oral dose of fosfomycin or a 5 day course of nitrofurantoin, response rates and adverse event rates were similar and there were "no clinically significant changes in biochemical or hematologic values").
- Durupt S, Josserand RN, Sibille M, Durieu I. Acute, recurrent fosfomycin-induced liver toxicity in an adult patient with cystic fibrosis. Scand J Infect Dis 2001; 33: 391-2. [PubMed: 11440232](30 year old woman with cystic fibrosis developed abdominal pain 4 days after starting intravenous fosfomycin [12 g daily] and imipenem [bilirubin not given, ALT 921 U/L, GGT 214 U/L], which resolved a month after antibiotics were stopped and recurred 3 days after starting cefepim and fosfomycin [ALT 163 U/L, GGT 79 U/L], resolving within 1 week of stopping and recurring with a third course of fosfomycin).
- Matsumori A, Yoneda S, Kobayashi Y, Takeda K, Andoh M, Yamane Y, Nishimura K, et al. [A case of acute severe hepatitis induced by fosfomycin]. Nihon Shokakibyo Gakkai Zasshi 2005; 102: 1207-11. Japanese. [PubMed: 16180681](30 year old man with an upper respiratory tract infection developed jaundice 3 days after a single oral dose of fosfomycin [bilirubin 6.5 mg/dL, ALT 558 U/L, Alk P 649 U/L, prothrombin index 44%], with a severe course, but resolving within 4 months: Case 1).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US between 2004 and 2008, more than 100 agents were implicated and the most commonly implicated class of agents was antibiotics, but no case was attributed to fosfomycin).
- Wang YP, Shi B, Chen YX, Xu J, Jiang CF, Xie WF. Drug-induced liver disease: an 8-year study of patients from one gastroenterological department. J Dig Dis 2009; 10: 195-200. [PubMed: 19659787](30 cases of drug induced liver injury were seen at a single large hospital in Shanghai between 2000 and 2007, including one case in a 50 year old man who developed mild hepatitis [bilirubin 2.5 mg/dL, ALT 12.8 times and Alk P 1.9 times ULN] within 3 days of starting fosfomycin).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to fosfomycin).
- Falagas ME, Vouloumanou EK, Togias AG, Karadima M, Kapaskelis AM, Rafailidis PI, Athanasiou S. Fosfomycin versus other antibiotics for the treatment of cystitis: a meta-analysis of randomized controlled trials. J Antimicrob Chemother 2010; 65: 1862-77. [PubMed: 20587612](Systematic review of 27 controlled trials of fosfomycin for urinary tract infections mentions diarrhea and headache as complications, but does not mention ALT elevations or hepatotoxicity).
- Qiao LD, Zheng B, Chen S, Yang Y, Zhang K, Guo HF, Yang B, et al. Evaluation of three-dose fosfomycin tromethamine in the treatment of patients with urinary tract infections: an uncontrolled, open-label, multicentre study. BMJ Open 2013; 3: e004157. [PMC free article: PMC3855495] [PubMed: 24309172](Among 356 Chinese patients with acute cystitis who received three 3 g doses of fosfomycin, side effects included diarrhea [5%]; no mention of ALT elevations or hepatotoxicity).
- Del RíA, Gasch O, Moreno A, Peñ, Cuquet J, Soy D, Mestres CA, et al.; FOSIMI Investigators. Efficacy and safety of fosfomycin plus imipenem as rescue therapy for complicated bacteremia and endocarditis due to methicillin-resistant Staphylococcus aureus: A multicenter clinical trial. Clin Infect Dis 2014; 59: 1105-12. [PubMed: 25048851](Among 16 patients with complicated MRSA infections treated with the combination of imipenem and fosfomycin [2 g intravenously every 6 hours for 4 to 75 days], blood cultures were negative at 72 hours in all patients and no breakthrough infections occurred; side effects included sodium overload in 3 patients and leukopenia in one; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. PubMed Citation. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 cases [36%] were attributed to antibiotics, but none to fosfomycin).
- Iarikov D, Wassel R, Farley J, Nambiar S. Adverse Events Associated with Fosfomycin Use: Review of the literature and analyses of the FDA adverse event reporting System database. Infect Dis Ther 2015; 4: 433-58. [PMC free article: PMC4675770] [PubMed: 26437630](Systematic review of the literature and the FDA reporting sytem for adverse events related to fosfomycin, found ALT elevations occurring in similar proportions of patients treated with fosfomycin (oral or iv) as with comparator antibiotics [<1% vs <1%] and that only 5 instances of hepatitis in patients receiving fosfomycin have been reported to the FDA, suggesting that liver injury is quite rare even with parenteral therapy).
- Matthews PC, Barrett LK, Warren S, Stoesser N, Snelling M, Scarborough M, Jones N. Oral fosfomycin for treatment of urinary tract infection: a retrospective cohort study. BMC Infect Dis 2016; 16: 556. [PMC free article: PMC5057270] [PubMed: 27729016](Among 75 patients treated for 151 episodes of urinary tract infections, adverse events were uncommon; no mention of ALT elevations or hepatotoxicity).
- Jacobson S, Junco Noa L, Ahmed S, Wallace MR. Efficacy and wafety of oral fosfomycin for urinary tract infections in hospitalized patients. Antimicrob Agents Chemother 2016; 60: 1952. [PMC free article: PMC4775933] [PubMed: 26921414](Among 71 patients treated with oral fosfomycin for urinary tract infections, cure rate was 83% and adverse event rate 4%, which were mostly nausea and vomiting; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- [Efficiency of fosfomycin trometamol for treatment of acute uncomplicated cystitis].[Urologiia. 2018][Efficiency of fosfomycin trometamol for treatment of acute uncomplicated cystitis].Kuzmenko AV, Kuzmenko VV, Gyaurgiev TA. Urologiia. 2018 Dec; (6):70-75.
- Review Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections.[Drugs. 1997]Review Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections.Patel SS, Balfour JA, Bryson HM. Drugs. 1997 Apr; 53(4):637-56.
- [Experience of using the antibacterial drug Fosfomycin Esparma for the treatment of acute uncomplicated cystitis in women].[Urologiia. 2019][Experience of using the antibacterial drug Fosfomycin Esparma for the treatment of acute uncomplicated cystitis in women].Aboyan IA, Orlov YN, Voloshina OA, Orlov NV, Pavlov DS. Urologiia. 2019 Sep; (4):20-25.
- Review Recurrent uncomplicated cystitis in women: allowing patients to self-initiate antibiotic therapy.[Prescrire Int. 2014]Review Recurrent uncomplicated cystitis in women: allowing patients to self-initiate antibiotic therapy.. Prescrire Int. 2014 Feb; 23(146):47-9.
- Review Fosfomycin for UTIs.[Drug Ther Bull. 2016]Review Fosfomycin for UTIs.. Drug Ther Bull. 2016 Oct; 54(10):114-117.
- Fosfomycin - LiverToxFosfomycin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...