NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Flecainide is an oral antiarrhythmic agent that has been in use for several decades. Long term flecainide therapy is associated with a low rate of serum enzyme elevations and is a very rare cause of clinically apparent acute liver injury.
Background
Flecainide (fle kay' nide) is a benzamide derivative analogue of the local anesthetic procaine and has electrophysiological effects that resemble quinidine (antiarrhythmic Class IC). Flecainide appears to act by blocking open sodium channels and outward potassium channels. As a consequence, it decreases cardiac automaticity, increases refractory periods and slows conduction. Flecainide was approved for use in the United States in 1985. Its current indications include prevention and suppression of life threatening ventricular arrhythmias and conversion of supraventricular tachyarrhythmias and subsequent maintenance of normal sinus rhythm in patients without structural heart disease, in whom other agents were unsuccessful. Flecainide is available in tablets of 50, 100 and 150 mg generically and under the brand name Tambocor. The usual maintenance dose in adults is 50 to 200 mg twice daily. The most common side effects include dizziness, visual blurring, headache, fatigue, anxiety, gastrointestinal upset and nausea.
Hepatotoxicity
In clinical trials, flecainide was associated with a low rate of serum aminotransferase and alkaline phosphatase elevations. Despite wide scale use, flecainide has only rarely been linked to cases of clinically apparent liver injury. The typical presentation is with a cholestatic hepatitis arising within 1 to 6 weeks of starting flecainide. In addition, instances of acute hepatic injury arising within 1 to 3 days of starting flecainide with marked, but short lived elevations in serum aminotransferase levels and minimal increases in alkaline phosphatase have been published, but may actually represent acute worsening of congestive heart failure and ischemic hepatitis due to the proarrhythmic effects of flecainide. In all instances, the liver injury was self limited. Immunoallergic and autoimmune features were not present.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which flecainide might cause liver injury is unknown. Flecainide is metabolized in the liver predominantly by CYP 2D6. In some instances, symptoms and liver test abnormalities may be due to worsening of congestive heart failure and hepatic congestion.
Outcome and Management
Liver injury due to flecainide is rare and usually mild. Cases of prolonged jaundice, but no reports of acute liver failure, chronic hepatitis or vanishing bile duct syndrome attributed to flecainide, have been published. There is little information about cross sensitivity to liver injury between flecainide and other oral antiarrhythmics, but such shared sensitivity is unlikely.
Drug Class: Antiarrhythmic Agents
CASE REPORT
Case 1. Cholestatic hepatitis due to flecainide.
[Modified from: Mikloweit P, Bienmüller H. [Drug-induced intrahepatic cholestasis caused by flecainide acetate and enalapril]. Internist (Berl) 1987; 28: 193-5. German. PubMed Citation]
A 64 year old man developed abnormal liver tests five weeks after starting flecainide for cardiac tachyarrhythmias. He had a history of coronary artery disease and had been hospitalized for acute myocardial infarction, during which he had several episodes of ventricular tachycardia treated with lidocaine and then with oral flecainide (200 mg daily). His other medications included furosemide, spironolactone, metoprolol, glibecamide, and coumadin. Five weeks after starting flecainide therapy, laboratory testing demonstrated a total serum bilirubin of 3.0 mg/dL and alkaline phosphatase 310 U/L (Table). Flecainide was stopped the following day, but serum alkaline phosphatase levels continued to rise. He was started on propafenone. Tests for hepatitis B were negative and ultrasonography showed no evidence of biliary obstruction. A liver biopsy showed a cholestatic hepatitis with portal inflammation. Thereafter, serum bilirubin levels and alkaline phosphatase levels began to improve. Limited follow up was provided. [A second case of cholestatic hepatitis in this publication was attributed to enalapril]
Key Points
| Medication: | Flecainide (200 mg daily) |
|---|---|
| Pattern: | Cholestatic (R=0.7) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 5 weeks |
| Recovery: | Unclear |
| Other medications: | Glyburide, metoprolol, furosemide, spironolactone, warfarin, and lidocaine |
Laboratory Values
Comment
This patient developed cholestatic hepatitis 5 weeks after starting flecainide with improvements starting within a few days of stopping therapy. The cholestatic picture, timing of onset and lack of other known hepatotoxic exposures make flecainide a likely cause.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Flecainide – Generic, Tambocor®
DRUG CLASS
Antiarrhythmic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
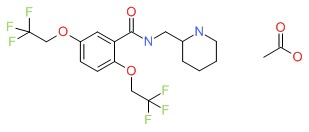
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Flecainide | 54143-56-5 | C17-H20-F6-N2-O3.C2-H4-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 24 Janurary 2018
- Zimmerman HJ. Antiarrhythmics. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 642-4.(Expert review of hepatotoxicity of antiarrhythmics and procainamide published in 1999; mentions that flecainide has been linked to rare cases of cholestatic jaundice).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40. (Review of hepatotoxicity of cardiovascular agents, mentions that.flecainide has been linked to rare cases of hepatocellular injury that is rapidly reversible).
- Sampson KJ, Kass RS. Antiarrhythmic drugs. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 815-48.(Textbook of pharmacology and therapeutics).
- Hopmann G, Surmann T. [Cholestatic jaundice during flecainide therapy]. Dtsch Med Wochenschr 1984; 109: 1863. German. [PubMed: 6499688](61 year old man developed nausea followed by jaundice 30 days after starting flecainide for ventricular arrhythmias [peak bilirubin 15.8 mg/dL, ALT 71 U/L, Alk P 585 U/L] with slow recovery, symptoms resolving after 2, jaundice 3.5 and liver enzymes 5 months after stopping).
- Mikloweit P, Bienmüller H. [Drug-induced intrahepatic cholestasis caused by flecainide acetate and enalapril]. Internist (Berl) 1987; 28: 193-5. German. [PubMed: 3034817](64 year old man developed jaundice 5 weeks after starting flecainide [peak bilirubin 7.6 mg/dL, ALT 152 U/L, Alk P 479 U/L, eosinophils 6%], resolving upon switching to propafenone: Case 1).
- Kühlkamp V, Haasis R, Seipel L. [Flecainide-induced hepatitis]. Z Kardiol 1988; 77: 678-80. German. [PubMed: 3149084](51 year old man developed nausea and abdominal pain 2 days after starting flecainide [ALT 993 U/L, bilirubin 3.0 mg/dL, Alk P normal], resolving within 2 weeks of stopping).
- Flecainide for supraventricular tachyarrhythmias. Med Lett Drugs Ther 1992; 34: 71-2. [PubMed: 1630411](Short summary of efficacy and safety of flecainide published soon after its approval in the US; adverse effects include dizziness and blurred vision, but recommended restricted use because of its proarrhythmic effects).
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991; 324: 781-8. [PubMed: 1900101](Among 1498 patients with myocardial infarction and ventricular ectopy, 323 were treated with flecainide and 318 with placebo and followed for a mean of 10 months; there was an excess mortality from cardiac arrest in antiarrhythmic treated patients; no mention of hepatotoxicity or ALT elevations).
- Roden DM. Antiarrhythmic drugs: from mechanisms to clinical practice. Heart 2000; 84: 339-46. [PMC free article: PMC1760959] [PubMed: 10956304](Overview of antiarrhythmic drugs which are separated into four classes based upon molecular target: I being sodium channel blockers; II beta blockers; III potassium channel blockers; and, IV calcium channel blockers; some agents having multiple targets).
- McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med 2003; 139: 1018-33. [PubMed: 14678922](Systematic review of literature on efficacy and safety of drugs for atrial fibrillation; flecainide has been shown to be effective in conversion of atrial fibrillation to normal sinus rhythm; hepatic side effects are not discussed).
- Drugs for cardiac arrhythmias. Treat Guidel Med Lett 2007; 5: 51-8. [PubMed: 17505408](Concise review of drugs for arrhythmias; flecainide is effective in preventing supraventricular tachycardia and atrial fibrillation in “otherwise healthy hearts” and in suppressing ventricular arrhythmias, but has been associated with an increase in mortality after myocardial infarction; no mention of hepatotoxicity as an adverse event).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34 . [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to flecainide).
- Tamargo J, Capucci A, Mabo P. Safety of flecainide. Drug Saf 2012; 35: 273-89. [PubMed: 22435343](Review of the use and safety of flecainide mentions rare reports of serum aminotransferase elevations and hepatic dysfunction, including cholestasis and hepatic failure: "However, no causal relationship has been established").
- Gao S, Dai W, Zhang L, Juhaeri J, Wang Y, Caubel P. Risk of Cardiovascular Events, Stroke, Congestive Heart Failure, Interstitial Lung Disease, and Acute Liver Injury: Dronedarone versus Amiodarone and Other Antiarrhythmics. J Atr Fibrillation 2013; 6: 890. [PMC free article: PMC5153130] [PubMed: 28496906](Analysis of health care database of 10,455 adults with atrial fibrillation or flutter seen between 2009 and 2010 treated with antiarrhythmics for serious outcomes found no difference in incidence [per 1000 person years] of acute liver injury for flecainide [5], dronedarone [7.6], propafenone [14] or even amiodarone [18.9]).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. PubMed Citation. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to flecainide).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. PubMed Citation. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were due to flecainide).
- Jenkins DJA, Freeman M, Mangat I, Srichaikul K, Jayalath VH, Faulkner D, Sievenpiper JL, et al. Flecainide and elevated liver enzymes in α1-antitrypsin deficiency. HeartRhythm Case Rep 2016; 2: 237-240. [PMC free article: PMC5419749] [PubMed: 28491677](72 year old man with atrial fibrillation developed abdominal discomfort and liver test abnormalities within days of starting metoprolol and flecainide [ALT ~200 U/L], which resolved within 10 days of stopping and recurred [ALT 60 U/L] with restarting flecainide).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Disopyramide.[LiverTox: Clinical and Researc...]Review Disopyramide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Predicting mortality after myocardial infarction from the response of RR variability to antiarrhythmic drug therapy.[J Am Coll Cardiol. 1994]Predicting mortality after myocardial infarction from the response of RR variability to antiarrhythmic drug therapy.Bigger JT Jr, Rolnitzky LM, Steinman RC, Fleiss JL. J Am Coll Cardiol. 1994 Mar 1; 23(3):733-40.
- Review Mexiletine.[LiverTox: Clinical and Researc...]Review Mexiletine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- [Flecainide: a new antiarrhythmic agent].[Arch Mal Coeur Vaiss. 1983][Flecainide: a new antiarrhythmic agent].Leclercq JF, Coumel P. Arch Mal Coeur Vaiss. 1983 Oct; 76(10):1218-30.
- A comparison of the antiarrhythmic effects on AV junctional re-entrant tachycardia of oral and intravenous flecainide acetate.[Eur Heart J. 1983]A comparison of the antiarrhythmic effects on AV junctional re-entrant tachycardia of oral and intravenous flecainide acetate.Bexton RS, Hellestrand KJ, Nathan AW, Spurrell RA, Camm AJ. Eur Heart J. 1983 Feb; 4(2):92-102.
- Flecainide - LiverToxFlecainide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...