NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Febuxostat is a newly introduced nonpurine xanthine oxidase inhibitor used for the treatment of gout. Chronic febuxostat therapy has been associated with minor serum aminotransferase elevations, but has yet to be linked to cases of clinically apparent acute liver injury.
Background
Febuxostat (fe bux' oh stat) is a nonpurine inhibitor of xanthine oxidase that shares no structural homology to allopurinol or to hypoxanthine. Therapy with febuxostat leads to lowering of serum uric acid levels within a few weeks, and chronic therapy has been shown to decrease uric acid levels into target levels of <6 mg/dL and to decrease acute gouty attacks. Febuxostat was approved for use in Europe in 2008 and in the United States in 2009. Current indications include therapy and prevention of gout, uric acid nephropathy, and the hyperuricemia caused by malignancy and anticancer therapy. Febuxostat is not recommended as therapy of asymptomatic hyperuricemia. Febuxostat is available in tablets of 40 and 80 mg under the brand names of Uloric and Adenuric. The recommended initial dose for therapy of gout is 40 mg daily, which can be increased to 80 mg daily to achieve uric acid levels below 6 mg/dL. Common side effects include nausea, diarrhea, dizziness and precipitation of acute gout for which reason it is often given in combination with colchicine for the first few months of treatment.
Hepatotoxicity
Liver test abnormalities have been reported to occur in 2% to 13% (average ~3.5%) of patients receiving febuxostat, but the levels are generally mild-to-moderate and self-limited. The height, nature and timing of these abnormalities have not been described. However, liver test elevations were the major reason for febuxostat discontinuation for adverse events (~2%) in clinical trials, despite the fact that no cases of jaundice or acute hepatitis were reported. Since its approval and more wide-scale use, there have been several individual case reports of liver injury attributed to febuxostat. Most cases have been marked by serum aminotransferase elevations without jaundice arising within days of starting febuxostat, including enzyme elevations in the setting of DRESS syndrome. At least one instance of a mixed-cholestatic hepatitis without immunoallergic features, arising after several months of treatment has been described. The product label for febuxostat lists hepatic steatosis, hepatitis and hepatomegaly as potential side effects. Furthermore, several cases of acute liver failure during febuxostat therapy have been reported to pharmacovigilance databases. Another unrelated, nonpurine xanthine oxidase inhibitor (benzbromarone) was not approved for use in the United States because of its potential for hepatic toxicity.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of febuxostat hepatotoxicity is believed to be due to its hepatic metabolism, the major pathway being glucuronidation with minor metabolism via the CYP 450 system.
Outcome and Management
The minor liver test abnormalities are reported to be self-limited, resolving with stopping the drug and, in many instances, resolving rapidly even with drug continuation. No instances of acute liver failure or chronic liver injury have been reported due to febuxostat, but the clinical experience with this agent is limited.
Drug Class: Antigout Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Febuxostat – Uloric®
DRUG CLASS
Antigout Agents/Gout Suppressants
Product labeling at DailyMed, National Library of Medicine, NIH
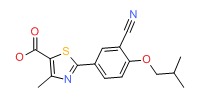
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Febuxostat | 144060-53-7 | C16-H16-N2-O3-S |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 29 January 2018
- Grosser T, Smyth E, FitzGerald GA. Pharmacology of gout. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 994-1004.(Textbook of pharmacology and therapeutics).
- Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Palo WA, Eustace D, Vernillet L, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005; 353: 2450-61. [PubMed: 16339094](762 patients at 112 North American centers received either allopurinol [300 mg/day] or febuxostat [80, 120 or 240 mg/day] for 52 weeks; reduction of uric acid to <6 mg/dL achieved in 53-62% of febuxostat- vs 21% of allopurinol-treated patients; rates of acute gout were similar; liver test abnormalities occurred in 4-5% of febuxostat vs 4% of allopurinol recipients; most common cause of discontinuation [2.3%]).
- Becker MA, Schumacher HR, Wortmann RL, MacDonald PA, Palo WA, Eustace D, Vernillet L, Joseph-Ridge N. Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase. A twenty-eight-day, multicenter, phase II randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout. Arthr Rheum 2005; 52: 916-23. [PubMed: 15751090](153 patients were randomized to febuxostat [40, 80 or 120 mg/day] or placebo for 28 days; fall of uric acid to <6 mg/dL occurred in 56-94% of treated but none on placebo; abnormal liver tests arose in 9.5% of febuxostat, but none of placebo-recipients).
- Bruce SP. Febuxostat: a selective xanthine oxidase inhibitor for the treatment of hyperuricemia and gout. Ann Pharmacother 2006; 40: 2187-94. [PubMed: 17132810](Review of pharmacology, mechanisms of action, clinical efficacy and side effects of febuxostat; liver test abnormalities occur in 7.5-13.5% of patients on febuxostat compared to 0% on placebo).
- Yu KH. Febuxostat: a novel non-purine selective inhibitor of xanthine oxidase for the treatment of hyperuricemia in gout. Recent Pat Inflamm Allergy Drug Discov 2007; 1: 69-75. [PubMed: 19075968](Critical review of literature on febuxostat).
- Schumacher HR Jr, Becker MA, Wortmann RL, MacDonald PA, Hunt B, Streit J, Lademacher C, Joseph-Ridge N. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double- blind, parallel-group trial. Arthritis Rheum 2008; 59: 1540-8. [PubMed: 18975369](1072 patients with gout randomized to febuxostat, allopurinol or placebo and treated for 28 weeks; abnormal liver tests [>1.5 times ULN] occurred in 4-6% on febuxostat, 2% placebo, and 6% allopurinol).
- Hair PI, McCormack PL, Keating GM. Febuxostat. Drugs 2008; 68: 1865-74. [PubMed: 18729537](Review of mechanism of action, pharmacokinetics, tolerability and efficacy of febuxostat; in pooled analyses, liver test elevations occurred in 3.5% of febuxostat treated [80 mg/day] leading to withdrawal in 2%, compared to 4.2% rate of liver test elevations and 0.5% withdrawals with allopurinol [300 mg/day]).
- Pascual E, Sivera F, Yasothan U, Kirkpatrick P. Febuxostat. Nat Rev Drug Discov 2009; 8: 191-2. [PubMed: 19247302](Brief review of febuxostat therapy in gout).
- Schumacher HR Jr, Becker MA, Lloyd E, MacDonald PA, Lademacher C. Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology (Oxford) 2009; 48:188-94. [PubMed: 19141576](Open label extension of phase 2 study [Becker 2005], of 116 patients with dose adjustment of 40-120 mg/day; gradual decrease in episodes of acute gout from 25/month to none, but also gradual increasing drop out rate [to 58 patients at 5 years]; liver test abnormalities in 13%, requiring discontinuation in 3%, no hepatitis or jaundice reported).
- Becker MA, Schumacher HR, Macdonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol 2009; 36; 1273-82. [PubMed: 19286847](1086 subjects enrolled in extension study of febuxostat or allopurinol for 31-40 months; ALT elevations ultimately required withdrawal in 9 of 801 patients [1.1%] on febuxostat [80 mg/day], two episodes of jaundice, but both thought to be due to unrelated biliary disease--stone and bile duct cancer).
- Becker MA, MacDonald PA, Hunt B, Gunawardhana L. Treating hyperuricemia of gout: safety and efficacy of febuxostat and allopurinol in older versus younger subjects. Nucleosides Nucleotides Nucleic Acids 2011; 30: 1011-7. [PubMed: 22132950](Reanalysis of a 6 month trial of febuxostat comparing 374 older [>65 years of age] to 1895 younger patients found ALT elevations were less common among older than younger patients [10% vs 3% were >2 times, 3% vs 1% >3 times ULN], and no patient developed jaundice in association with ALT elevations).
- Gray CL, Walters-Smith NE. Febuxostat for treatment of chronic gout. Am J Health Syst Pharm 2011; 68: 389-98. [PubMed: 21330679](Review of structure, mechanism of action, pharmacology, clinical efficacy and safety of febuxostat; the most commmon adverse event leading to discontinuation was ALT elevations, which occurred in 6.6% of recipients).
- Faruque LI, Ehteshami-Afshar A, Wiebe N, Tjosvold L, Homik J, Tonelli M. A systematic review and meta-analysis on the safety and efficacy of febuxostat versus allopurinol in chronic gout. Semin Arthritis Rheum 2013; 43: 367-75. [PubMed: 24326033](Review of efficacy and safety of febuxostat in comparison to allopurinol concludes that there is no evidence to justify the routine use of febuxostat at present; no discussion of hepatotoxicity or ALT elevations).
- Febuxostat: hepatic failure. Prescrire Int 2013; 22: 297. [PubMed: 24600733](News report mentions that 13 reports of acute liver failure during febuxostat therapy have been reported to the European pharmacovigilance databse, 6 of which were fatal).
- Huang X, Du H, Gu J, Zhao D, Jiang L, Li X, Zuo X, et al. An allopurinol-controlled, multicenter, randomized, double-blind, parallel between-group, comparative study of febuxostat in Chinese patients with gout and hyperuricemia. Int J Rheum Dis 2014; 17: 679-86. [PubMed: 24467549](Trial of two doses of febuxostat vs allopurinol in 512 Chinese patients with gout found similar rates of side effects, liver test abnormalities arising in 3.5% of allopurinol and 2.9% and 1.2% of febuxostat treated subjects; no mention of clinically apparent liver injury).
- Ito K, Ueda Y, Miyazawa H, Kaku Y, Hirai K, Hoshino T, Nabata A, et al. Acute severe liver dysfunction induced by febuxostat in a patient undergoing hemodialysis. CEN Case Rep 2014; 3: 158-61. [PMC free article: PMC5411563] [PubMed: 28509193](58 year old man with type 2 dfiabetes and chronic renal failure developed marked serum enzyme elevations within 2 days of starting hemodialysis and switching from allopurinol to febuxostat [ALT rising from 13 to 1134 U/L, Alk P and bilirubin normal], abnormalities resolving within 2 weeks of stopping).
- Chou HY, Chen CB, Cheng CY, Chen YA, Ng CY, Kuo KL, Chen WL, Chen CH. Febuxostat-associated drug reaction with eosinophilia and systemic symptoms (DRESS). J Clin Pharm Ther 2015; 40: 689-92. [PubMed: 26365588](81 year old Taiwanese man developed fever, severe rash, lymphadenopathy and facial edema without eosinophilia 2 days after starting febuxostat [ALT 66 rising to 210 U/L, bilirubin normal], resolving within 3 weeks of stopping).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 8 [0.9%] were attributed to agents used to treat gout, including 7 to allopurinol and 1 to febuxostat [Bohm 2016]).
- Bohm M, Vuppalanchi R, Chalasani N; Drug-Induced Liver Injury Network (DILIN). Febuxostat-induced acute liver injury. Hepatology 2016; 63: 1047-9. [PMC free article: PMC4764455] [PubMed: 26679098](34 year old man with gout developed jaundice 2 months after adding febuxostat to a regimen of allopurinol and colchicine [bilirubin 8.3 mg/dL, ALT 148 U/L, Alk P 201 U/L] with persistence of injury for over a month at the time of a normal ERCP, but then resolution within 3 weeks of stopping febuxostat).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Febuxostat: a selective xanthine-oxidase/xanthine-dehydrogenase inhibitor for the management of hyperuricemia in adults with gout.[Clin Ther. 2009]Review Febuxostat: a selective xanthine-oxidase/xanthine-dehydrogenase inhibitor for the management of hyperuricemia in adults with gout.Ernst ME, Fravel MA. Clin Ther. 2009 Nov; 31(11):2503-18.
- Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout.[Arthritis Rheum. 2005]Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout.Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Palo WA, Eustace D, Vernillet L, Joseph-Ridge N. Arthritis Rheum. 2005 Mar; 52(3):916-23.
- Review Febuxostat: a selective xanthine oxidase inhibitor for the treatment of hyperuricemia and gout.[Ann Pharmacother. 2006]Review Febuxostat: a selective xanthine oxidase inhibitor for the treatment of hyperuricemia and gout.Bruce SP. Ann Pharmacother. 2006 Dec; 40(12):2187-94. Epub 2006 Nov 28.
- The effect of age and gender on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase.[J Clin Pharmacol. 2008]The effect of age and gender on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase.Khosravan R, Kukulka MJ, Wu JT, Joseph-Ridge N, Vernillet L. J Clin Pharmacol. 2008 Sep; 48(9):1014-24. Epub 2008 Jul 17.
- Febuxostat: the evidence for its use in the treatment of hyperuricemia and gout.[Core Evid. 2010]Febuxostat: the evidence for its use in the treatment of hyperuricemia and gout.Gaffo AL, Saag KG. Core Evid. 2010 Jun 15; 4:25-36. Epub 2010 Jun 15.
- Febuxostat - LiverToxFebuxostat - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...