NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ezogabine, which is known as retigabine in Europe, is a unique anticonvulsant used largely as an adjunctive agent in the treatment of partial seizures. Therapy with ezogabine has not been associated with serum aminotransferase elevations, and clinically apparent liver injury from ezogabine has yet to be reported and must be rare, if it occurs at all.
Background
Ezogabine (e zog' a been) is an anticonvulsant with a unique mechanism of action, decreasing excitability and seizure activity by opening voltage-gated potassium channels in the brain. Ezogabine has been shown to be effective both as monotherapy and in combination with other anticonvulsants for partial seizures. Ezogabine was approved for use in the United States in 2011 and current indications are as adjunctive therapy for partial seizures. Ezogabine is available in tablets of 50, 200, 300 and 400 mg under the brand name Potiga in the United States and Trobalt in Europe and elsewhere. The recommended initial dose in adults is 100 mg three times daily, which can be increased to 200 to 400 mg three times daily. The dose should be increased and tapered gradually. The most common side effects are dose related and include dizziness, somnolence, impaired concentration, nervousness, headache, fatigue nausea, weakness and tremor. Long term therapy has been associated with urinary retention and blue discoloration of the skin, lips, sclera and retina. Rare, but potentially severe adverse events include psychiatric symptoms such as confusion and hallucination and decrease in visual acuity as a result of retinal pigmentation.
Hepatotoxicity
Limited data are available on the hepatotoxicity of ezogabine. In clinical trials, therapy with ezogabine was not associated with an increased frequency of serum aminotransferase elevations as compared to placebo treatment, and there were no instances of clinically apparent liver injury. No individual case reports of ezogabine hepatotoxicity have been published since its more wide spread clinical availability. Thus, clinically apparent liver injury due to ezogabine must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Ezogabine is extensively metabolized by the liver, largely by glucuronidation and acetylation. However, its metabolism is independent of microsomal P450 system and it has no effect on P450 activity and lacks significant drug-drug interactions.
Drug Class: Anticonvulsants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ezogabine – Potiga®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ezogabine | 150812-12-7 | C16-H18-F-N3-O2 |
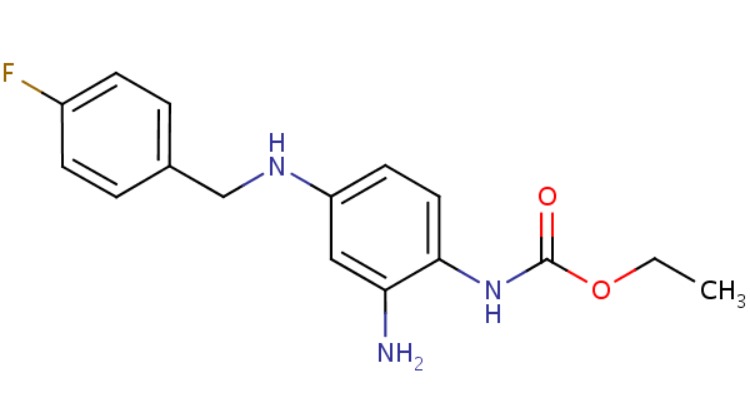 |
ANNOTATED BIBLIOGRAPHY
References updated: 18 February 2018
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999; ezogabine is not discussed).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-42.(Review of anticonvulsant induced liver injury; ezogabine is not discussed).
- McNamara JO. Pharmacology of the epilepsies. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman.s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 583-607.(Textbook of pharmacology and therapeutics).
- Porter RJ, Partiot A, Sachdeo R, Nohria V, Alves WM; 205 Study Group. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology 2007; 68: 1197-204. [PubMed: 17420403](Among 309 patients with partial seizures treated for 16 weeks with one of 3 doses of ezogabine or placebo, side effects were largely CNS related and "there were no clinically relevant findings related to ... clinical laboratory measurements").
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, valproate accounted for 6 cases, lamotrigine 5, phenytoin 5, gabapentin and topiramate 1 each; none were due to ezogabine).
- Brodie MJ, Lerche H, Gil-Nagel A, Elger C, Hall S, Shin P, Nohria V, et al.; RESTORE 2 Study Group. Efficacy and safety of adjunctive ezogabine(retigabine) in refractory partial epilepsy. Neurology 2010; 75: 1817-24. [PubMed: 20944074](Among 538 patients with partial seizures treated with two doses of ezogabine or placebo, adverse events were mild-to-moderate in severity, possibly dose related and largely CNS related; ALT elevations above 3 times ULN occurred in 2.5% of ezogabine vs 3.9% of placebo recipients).
- French JA, Abou-Khalil BW, Leroy RF, Yacubian EM, Shin P, Hall S, Mansbach H, et al; RESTORE 1/Study 301 Investigators. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology 2011; 76: 1555-63. [PubMed: 21451152](Among 305 patients with partial seizures treated with ezogabine or placebo for 18 weeks, there were "...no clinically significant abnormal results or trends in laboratory values").
- Knowles SR, Dewhurst N, Shear NH. Anticonvulsant hypersensitivity syndrome: an update. Expert Opin Drug Saf 2012; 11: 767-78. [PubMed: 22794330](Updated review of anticonvulsant hypersensitivity syndrome which is associated with phenytoin, phenobarbital, lamotrigine and carbamazepine, and rarely with zonisamide, valproate and oxcarbazepine; ezogabine is not mentioned).
- Yamada M, Welty TE. Ezogabine: an evaluation of its efficacy and safety as adjunctive therapy for partial-onset seizures in adults. Ann Pharmacother 2012; 46: 1358-67. [PubMed: 22991134](Systematic review of efficacy and safety of ezogabine states that in 3 large clinical trials side effects were generally mild-to-moderate [dizziness, somnolence, confusion, fatigue, headache and dysarthria]; no mention of ALT elevations or hepatotoxicity).
- Gil-Nagel A, Brodie MJ, Leroy R, Cyr T, Hall S, Castiglia M, Twomey C, VanLandingham K. Safety and efficacy of ezogabine (retigabine) in adults with refractory partial-onset seizures: Interim results from two ongoing open-label studies. Epilepsy Res 2012; 102 (1-2): 117-21. [PubMed: 22771137](Among 336 patients with partial seizures treated with ezogabine in 2 open label studies, 3% had urinary retention; no mention of ALT elevations or hepatotoxicity).
- Drugs for epilepsy. Treat Guidel Med Lett 2013; 11: 9-18. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants; tiagabine is approved only for treatment of partial seizures and has prominent gastrointestinal and CNS side effects; no mention of hepatotoxicity).
- Faulkner MA, Burke RA. Safety profile of two novel antiepileptic agents approved for the treatment of refractory partial seizures: ezogabine (retigabine) and perampanel. Expert Opin Drug Saf 2013; 12: 847-55. [PubMed: 23883095](Review of safety of ezogabine from 3 phase III studies states that "no negative laboratory, ECG or vital sign events were documented").
- Biton V, Gil-Nagel A, Brodie MJ, Derossett SE, Nohria V. Safety and tolerability of different titration rates of retigabine (ezogabine) in patients with partial-onset seizures. Epilepsy Res 2013; 107: 138-45. [PubMed: 24094693](Among 77 patients with partial onset seizures treated with ezogabine for up to 50 days, "...changes from baseline in clinical laboratory parameters ...were not clinically relevant").
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were attributed to anticonvulsants, but none specifically to ezogabine).
- Lerche H, Daniluk J, Lotay N, DeRossett S, Edwards S, Brandt C. Efficacy and safety of ezogabine/retigabine as adjunctive therapy to specified single antiepileptic medications in an open-label study of adults with partial-onset seizures. Seizure 2015; 30: 93-100. [PubMed: 26216692](Among 203 patients with partial onset seizures and inadequate control on a single anticonvulsant who added ezogabine using flexible doses, the response rate was 54% while common adverse events were dizziness [26%], somnolence [21%] and fatigue [8%], but there were "no clinically significant changes in hematology or clinical chemistry data from baseline").
- Clark S, Antell A, Kaufman K. New antiepileptic medication linked to blue discoloration of the skin and eyes. Ther Adv Drug Saf 2015; 6: 15-9. [PMC free article: PMC4308410] [PubMed: 25642319](Review of problematic adverse events of ezogabine which have led to an FDA alert including urinary retention [probably due to its effects on potassium channels in the bladder], prolongation of QTc interval, psychiatric symptoms such as confusion, euphoria, and hallucinations, and blue-gray discoloration of skin, lips, nails, sclera and retina which cause decrease in visual acuity especially with prolonged use; no mention of ALT elevations or hepatotoxicity).
- Lim KS, Lotay N, White R, Kwan P. Efficacy and safety of retigabine/ezogabine as adjunctive therapy in adult Asian patients with drug-resistant partial-onset seizures: A randomized, placebo-controlled Phase III study. Epilepsy Behav 2016; 61: 224-30. [PubMed: 27376872](Among 75 Asian adults with partial onset seizures treated with ezogabine [600 or 900 mg] or placebo once daily for 16 weeks, response rates were 31% and 17% with ezogabine vs 0% with placebo and there were "no safety concerns" and no mention of ALT elevations or hepatotoxicity).
- Zaccara G, Giovannelli F, Giorgi FS, Franco V, Gasparini S, Benedetto U. Tolerability of new antiepileptic drugs: a network meta-analysis. Eur J Clin Pharmacol 2017; 73: 811-7. [PubMed: 28378057](Metaanalysis of the comparative tolerability of 18 new anticonvulsant agents including tiagabine but not ezogabine, found lowest rates of withdrawal for adverse events with levetiracetam, brivaracetam, and gabapentin with lowest tolerability from eslicarbazepine, oxcarbazepine, lacosamide and topiramate; tiagabine had an intermediate level of tolerability).
- Drugs for epilepsy. Med Lett Drugs Ther 2017; 59 (1526): 121-30. [PubMed: 28746301](Concise review of the drugs available for therapy of epilepsy does not list ezogabine as either a first or second line drug for epilepsy and does not discuss it as a specific agent).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Retigabine (ezogabine) as add-on therapy for partial-onset seizures: an update for clinicians.[Ther Adv Chronic Dis. 2011]Retigabine (ezogabine) as add-on therapy for partial-onset seizures: an update for clinicians.Harris JA, Murphy JA. Ther Adv Chronic Dis. 2011 Nov; 2(6):371-6.
- Review Ezogabine (retigabine) and its role in the treatment of partial-onset seizures: a review.[Clin Ther. 2012]Review Ezogabine (retigabine) and its role in the treatment of partial-onset seizures: a review.Splinter MY. Clin Ther. 2012 Sep; 34(9):1845-56.e1. Epub 2012 Aug 21.
- Review Efficacy of retigabine in adjunctive treatment of partial onset seizures in adults.[J Cent Nerv Syst Dis. 2013]Review Efficacy of retigabine in adjunctive treatment of partial onset seizures in adults.Splinter MY. J Cent Nerv Syst Dis. 2013 Oct 23; 5:31-41. Epub 2013 Oct 23.
- Profile of ezogabine (retigabine) and its potential as an adjunctive treatment for patients with partial-onset seizures.[Neuropsychiatr Dis Treat. 2011]Profile of ezogabine (retigabine) and its potential as an adjunctive treatment for patients with partial-onset seizures.Weisenberg JL, Wong M. Neuropsychiatr Dis Treat. 2011; 7:409-14. Epub 2011 Jul 7.
- Efficacy and tolerability exposure-response relationship of retigabine (ezogabine) immediate-release tablets in patients with partial-onset seizures.[Clin Ther. 2013]Efficacy and tolerability exposure-response relationship of retigabine (ezogabine) immediate-release tablets in patients with partial-onset seizures.Tompson DJ, Crean CS, Reeve R, Berry NS. Clin Ther. 2013 Aug; 35(8):1174-1185.e4. Epub 2013 Aug 1.
- Ezogabine - LiverToxEzogabine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...