NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ezetimibe is an inhibitor of intestinal cholesterol absorption that is widely used in the therapy of hypercholesterolemia, usually in combination with other agents. Ezetimibe therapy is associated with a low rate of serum aminotransferase elevations and clinically apparent liver injury due to ezetimibe occurs, but is rare.
Background
Ezetimibe (e zet' i mibe) is a synthetic azetidinone that inhibits cholesterol absorption from the intestine. Ezetimibe acts by binding to and inhibiting the Niemann-Pick C1-like 1 protein (NPC1), the major transporter of cholesterol in the intestinal brush border. Ezetimibe by itself lowers serum cholesterol, but the inhibition of cholesterol absorption is usually followed by a compensatory increase in hepatic cholesterol synthesis, a response that can be blocked by hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors [statins]. For this reason, ezetimibe is commonly used in combination with a statin. Addition of ezetimibe to a maximized dose of statins generally lowers LDL-cholesterol levels by 22% to 24%. Ezetimibe was approved for use in the United States in 2002 and is usually recommended as an adjunctive therapy to diet or in combination with a statin in management of patients with hypercholesterolemia at high risk for cardiovascular or cerebrovascular disease. It is also used alone in patients who are intolerant of statins and can be combined with other lipid-lowering agents (such as fibrates or anti-PCSK9 monoclonal antibodies). Ezetimibe is available in 10 mg tablets generically and under the brand name Zetia, and the usually recommended dose is 10 mg daily. Ezetimibe is also available in a fixed combination with simvastatin (20 or 40 mg) under the trade name Vytorin and with atorvastatin (10, 20, 40 or 80 mg) under the trade name Liptruzet. Ezetimibe has achieved wide scale acceptance and several million prescriptions are filled yearly. Common side effects of ezetimibe include diarrhea and flatulence. Rare, potentially severe adverse effects include hypersensitivity reactions, myopathy and rhabdomyolysis.
Hepatotoxicity
Therapy with ezetimibe alone or in combination with other lipid lowering agents is associated with a low rate of serum enzyme elevations (0.5% to 1.5%), but most elevations are self-limited and not associated with jaundice or symptoms. In large randomized controlled trials, ezetimibe by itself has not been associated with a higher rate of serum ALT elevation than occurs with placebo therapy. However, the addition of ezetimibe to statin therapy has been associated with a slight increase in the likelihood of serum aminotransferase elevations or rates of discontinuation due to liver test abnormalities. Clinically apparent acute liver injury due to ezetimibe has been reported, but is rare. Furthermore, because this agent is often used in combination with other cholesterol lowering drugs, the role of ezetimibe in these reports is not always well defined. The latency to onset of clinically apparent liver injury attributed to ezetimibe has ranged from 2 to 10 months and the pattern of serum enzyme elevations has ranged from hepatocellular to cholestatic. Cases of autoimmune hepatitis-like injury have been described in patients taking the combination of ezetimibe and a statin, and the role of ezetimibe in these reactions is difficult to assign (Case 1). A single instance of vanishing bile duct syndrome due to ezetimibe has been described in a patient who continued on ezetimibe for several months despite presence of jaundice.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
Ezetimibe is metabolized by the P450 system, but has little effect on the pharmacokinetics of other drugs, perhaps because it is rapidly conjugated and excreted in the bile as a glucuronide. Cases of an autoimmune hepatitis-like syndrome suggest immune mediated injury may be the cause of some clinically apparent liver injury due to ezetimibe.
Outcome and Management
The mild ALT elevations associated with ezetimibe therapy are usually self-limited and do not require dose modification. Cases of acute liver injury due to ezetimibe have usually been self-limited, but instances of vanishing bile duct syndrome, chronic autoimmune hepatitis and acute liver failure (Case 2) have been reported. Patients with autoimmune hepatitis-like liver injury due to ezetimibe may warrant corticosteroid therapy, but the dose and duration of therapy should be kept to a minimum. The product label for ezetimibe includes a warning about enzyme elevations during combination therapy and recommends screening for liver test abnormalities before starting treatment and monitoring tests during therapy if clinically indicated or elevations are suspected. Ezetimibe has a unique mechanism of action and chemical structure, and there is no reason to suspect cross sensitivity to liver injury between ezetimibe and other cholesterol lowering agents. Ezetimibe is often used in patients with intolerance to statins because of muscle pains and myopathy.
Drug Class: Antilipemic Agents
Other Drugs in the Subclass, Statins: Atorvastatin, Fluvastatin, Lovastatin, Pitavastatin, Pravastatin, Rosuvastatin, Simvastatin
CASE REPORTS
Case 1. Autoimmune hepatitis like injury arising during atorvastatin and ezetimibe therapy.(1)
A 50 year old woman developed nausea, abdominal pain and jaundice with features of autoimmune hepatitis during combination therapy with atorvastatin (80 mg daily for 16 months) and ezetimibe (10 mg daily for 3 months). There was no previous history of liver disease and laboratory tests had been normal shortly before ezetimibe was started. She had multiple other medical problems including coronary artery disease, osteoarthritis, hypothyroidism, and gastroesophageal reflux. She drank little alcohol and had no exposures to hepatitis. On presentation, she had epigastric tenderness but no rash or fever. Laboratory tests showed raised bilirubin and serum enzymes (Table). Both ezetimibe and atorvastatin were stopped. Tests for hepatitis A, B and C were negative and ultrasound imaging showed no evidence of biliary obstruction. She tested positive for antinuclear antibody and antibodies to double-stranded DNA (anti-dsDNA). A liver biopsy showed hepatitis with eosinophils and plasma cells. Over the ensuing few weeks, her symptoms resolved and laboratory tests improved. Six weeks after onset, her liver tests were normal and autoantibody levels had fallen. She was started on rosuvastatin for her hypercholesterolemia and did well without recurrence of symptoms or liver test abnormalities.
Key Points
| Medication: | Ezetimibe (10 mg daily) and atorvastatin (80 mg daily) |
|---|---|
| Pattern: | Hepatocellular (R=10.7) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 12 weeks for ezetimibe; 16 months for atorvastatin |
| Recovery: | ~6 weeks |
| Other medications: | Furosemide, diltiazem, diclofenac, acetaminophen, atenolol, ibuprofen, clopidogrel, thyroxine, lactulose, and nitrates |
Laboratory Values
| Time After Starting | Time After Stopping | AST (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | 17 | 75 | 0.5 | Atorvastatin 80 mg (13 months) | |
| 0 | Ezetimibe (10 mg/day) started | ||||
| 12 weeks | 0 | 1626 | 468 | 4.6 | ANA 1:160, anti-dsDNA 38 |
| 13 weeks | 10 days | 858 | 424 | 5.1 | ANA 1:80, anti-dsDNA 31 |
| 14 weeks | 15 days | 266 | 340 | 2.7 | |
| 18 weeks | 45 days | 20 | 100 | 0.6 | ANA 1:40, anti-dsDNA 6 |
| Normal Values | <42 | <130 | <1.2 | ||
Comment
A well documented example of drug induced autoimmune hepatitis, with resolution when atorvastatin and ezetimibe were stopped. Particularly convincing was the decrease in autoantibody titers with drug withdrawal. Ezetimibe is perhaps the most likely candidate to have caused the liver injury, but cases of autoimmune hepatitis-like injury from atorvastatin have been reported with latency to onset of more than a year.
Case 2. Acute liver failure attributed to simvastatin and ezetimibe therapy.(2)
A 70 year old woman developed abnormal liver tests ten weeks after switching from simvastatin (40 mg) monotherapy to the combination of simvastatin (40 mg) and ezetimibe (10 mg) daily. She had a history of coronary artery disease, hypertension, depression and hypercholesterolemia and had been on simvastatin for one and a half years with normal serum enzymes (Table). Despite stopping both simvastatin and ezetimibe when elevations in serum enzymes were first found, the patient developed jaundice and progressive symptoms of liver failure. Tests for hepatitis A, B and C were negative as were autoimmune markers. A liver biopsy showed changes suggestive of drug induced liver injury and severe cholestasis. She subsequently developed progressive encephalopathy and worsening coagulation indices, and was listed and underwent successful liver transplantation approximately 4 weeks after stopping therapy. In follow up for two years after transplantation, liver enzymes were normal.
Key Points
| Medication: | Ezetimibe and simvastatin |
|---|---|
| Pattern: | Hepatocellular (R=25) |
| Severity: | 5+ (emergency liver transplantation) |
| Latency: | 10 weeks to liver test abnormalities, 12 weeks to jaundice |
| Recovery: | None |
| Other medications: | Enalapril, escitalopram chronically |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | 19 | 65 | 0.5 | Before starting simvastatin | |
| 0 | 14 | 51 | 0.4 | On simvastatin before ezetimibe | |
| 10 weeks | 0 | 842 | 0.4 | Simvastatin & ezetimibe stopped | |
| 12 weeks | 2 weeks | 2595 | 217 | 15.8 | Admitted |
| 14 weeks | 4 weeks | 1951 | 116 | 21.6 | INR=2.0 |
| Emergency liver transplantation performed | |||||
| Post-op day 1 | 209 | 83 | 8.4 | ||
| Post-op day 23 | 39 | 82 | 1.0 | ||
| 2 years after transplant | 18 | 79 | 0.5 | ||
| Normal Values | <56 | <126 | <1.2 | ||
Comment
The onset of injury 10 weeks after starting ezetimibe suggests that it was the cause of the acute liver injury, but onset of hepatitis due to simvastatin can occur months to years after its initiation. Since ezetimibe is usually used in combination with a statin, it is usually difficult to attribute the liver injury to ezetimibe alone. This is one of the few case reports of acute liver failure due to a cholesterol lowering agent.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ezetimibe – Generic, Zetia®
DRUG CLASS
Antilipemic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
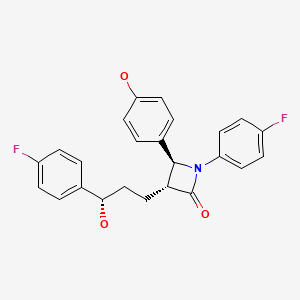
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ezetimibe | 163222-33-1 | C24- H21-F2-N-O3 |
|
CITED REFERENCES
- 1.
- van Heyningen C. Drug-induced acute autoimmune hepatitis during combination therapy with atorvastatin and ezetimibe. Ann Clin Biochem. 2005;42:402–4. [PubMed: 16168199]
- 2.
- Tuteja S, Pyrsopoulos NT, Wolowich WR, Khanmoradi K, Levi DM, Selvaggi G, Weisbaum G, et al. Simvastatin-ezetimibe-induced hepatic failure necessitating liver transplantation. Pharmacotherapy. 2008;28:1188–93. [PubMed: 18752389]
ANNOTATED BIBLIOGRAPHY
References updated: 01 December 2021
Abbreviations used: ANA, antinuclear antibody; HDL, high density lipoprotein; LDL, low density lipoprotein; OD, odds ratio.
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic medications. Lipid lowering agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of lipid lowering agents mentions that cases of severe cholestatic, as well as autoimmune, hepatitis have been linked to ezetimibe use).
- Gurgle H, Blumenthal DK. Drug therapy for dyslipidemias. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 605-618.(Textbook of pharmacology and therapeutics).
- Gagné C, Gaudet D, Bruckert E., Ezetimibe Study Group. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105:2469–75. [PubMed: 12034651](Prospective trial comparing the combination of ezetimibe and either atorvastatin or simvastatin to the statin alone in 50 patients with homozygous familial hypercholesterolemia; side effects were similar, one patients on combination therapy and one on statins alone had ALT elevations above 3 times ULN, but both resolved).
- Bays HE, Ose L, Fraser N, Tribble DL, Quinto K, Reyes R, Johnson-Levonas AO, et al. Ezetimibe Study Group. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2004;26:1758–73. [PubMed: 15639688](Controlled trial of various doses of ezetimibe, simvastatin or the combination vs placebo for 12 weeks in 1528 patients with hypercholesterolemia; confirmed ALT elevations above 3 times ULN occurred in 0.7% on placebo, 0.7% on ezetimibe alone, 1.1% on simvastatin alone and 1.5% on the combination).
- Masana L, Mata P, Gagné C, Sirah W, Cho M, Johnson-Levonas AO, Meehan A, et al. Ezetimibe Study Group. Long-term safety and, tolerability profiles and lipid-modifying efficacy of ezetimibe coadministered with ongoing simvastatin treatment: a multicenter, randomized, double-blind, placebo-controlled, 48-week extension study. Clin Ther. 2005;27:174–84. [PubMed: 15811480](Trial comparing addition of ezetimibe vs placebo to ongoing simvastatin therapy for hypercholesterolemia for up to 48 weeks; only one instance of ALT levels above 3 times ULN and no case of clinically apparent liver injury in 355 patients on the combination).
- Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44:467–94. [PubMed: 15871634](Review of biochemistry, pharmacology, mechanism of action, drug interactions of ezetimibe; ezetimibe is extensively conjugated to glucuronide in the intestine and liver and while it has inhibitory activity against CYP 3A4, it does not exhibit major drug-drug interactions with other agents that are metabolized by this pathway).
- van Heyningen C. Drug-induced acute autoimmune hepatitis during combination therapy with atorvastatin and ezetimibe. Ann Clin Biochem. 2005;42:402–4. [PubMed: 16168199](50 year old woman developed jaundice 16 months after starting atorvastatin and 3 months after starting ezetimibe therapy [bilirubin 4.6 mg/dL, AST 1626 U/L, Alk P 468 U/L, positive ANA and anti-dsDNA], resolving 6 weeks after stopping both: Case 1).
- Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. Am Heart J. 2005;149:464–73. [PubMed: 15864235](Controlled trial of ezetimibe/simvastatin vs atorvastatin in 1902 patients with hypercholesterolemia, ALT elevations above 3 times ULN occurred in 1.1% on atorvastatin and none on ezetimibe/simvastatin).
- Stolk MF, Becx MC, Kuypers KC, Seldenrijk CA. Severe hepatic side effects of ezetimibe. Clin Gastroenterol Hepatol. 2006;4:908–11. [PubMed: 16797241](Two patients with severe but clinically distinct liver injury; 52 year old woman developed jaundice 4 months after ezetimibe was added to atorvastatin therapy, but both were continued for another 6 months [bilirubin 5.4 mg/dL, ALT 179 U/L, Alk P 1345 U/L] followed by a chronic cholestatic course and biopsy showing bile duct paucity; 58 year old man developed abdominal pain and nausea 2 months after adding ezetimibe to chronic atorvastatin therapy [bilirubin 0.9 mg/dL, ALT 575 U/L, Alk P 116 U/L, SMA positive], responding ultimately to a course of prednisone).
- Goldberg RB, Guyton JR, Mazzone T, Weinstock RS, Polis A, Edwards P, Tomassini JE, et al. Ezetimibe/simvastatin vs atorvastatin in patients with type 2 diabetes mellitus and hypercholesterolemia: the VYTAL study. Mayo Clin Proc. 2006;81:1579–88. [PubMed: 17165637](Controlled trial of atorvastatin vs simvastatin/ezetimibe in 1229 patients with diabetes and hypercholesterolemia; confirmed ALT elevations above 3 times ULN occurred in 0.3% of atorvastatin vs no simvastatin/ezetimibe treated patients, and no clinically apparent liver injury).
- Bhardwah SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007;11:597–613. [PMC free article: PMC2048990] [PubMed: 17723922](Review of hepatotoxicity of lipid lowering agents; cases of acute cholestatic and autoimmune hepatitis-like injury from ezetimibe have been reported).
- Ose L, Johnson-Levonas A, Reyes R, Lin J, Shah A, Tribble D, Musliner T., Vytorin Extension Study Group. A multi-centre, randomised, double-blind 14-week extension study examining the long-term safety and efficacy profile of the ezetimibe/simvastatin combination tablet. Int J Clin Pract. 2007;61:1469–80. [PubMed: 17655686](Continuation of trial described by Bayes [2004] with 1104 patients continuing in an extension study of simvastatin vs simvastatin/ezetimibe in various dosages; confirmed ALT elevations above 3 times ULN occurred in 1.3% on simvastatin alone and 1.5% on the combination; no cases of clinically apparent liver injury).
- Tuteja S, Pyrsopoulos NT, Wolowich WR, Khanmoradi K, Levi DM, Selvaggi G, Weisbaum G, et al. Simvastatin-ezetimibe-induced hepatic failure necessitating liver transplantation. Pharmacotherapy. 2008;28:1188–93. [PubMed: 18752389](70 year old woman developed acute liver failure 10 weeks after addition of ezetimibe to long term simvastatin therapy [1.5 years] [bilirubin 0.4 rising to 23.7 mg/dL, ALT 842 to 2595 U/L, Alk P 217 U/L], INR rising to 2.0 and emergency liver transplant: Case 2).
- Liu Q, Tobias H, Petrovic LM. Drug-induced liver injury associated with ezetimibe therapy. Dig Dis Sci. 2007;52:602–5. [PubMed: 17219067](75 year old woman developed elevated liver tests 6 months after starting ezetimibe without a statin [bilirubin 0.9 mg/dL, ALT 731 U/L, Alk P 149 U/L, ANA 1:320], resolution within 4 months of stopping).
- Guyton JR, Brown BG, Fazio S, Polis A, Tomassini JE, Tershakovec AM. Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J Am Coll Cardiol. 2008;51:1564–72. [PubMed: 18420099](Controlled trial comparing niacin alone vs simvastatin/ ezetimibe vs all three for 24 weeks in 1220 patients with hypercholesterolemia; confirmed ALT elevations above 3 times ULN occurred in 0.4% on niacin, 0.4% on simvastatin/ezetimibe, and 0.5% on all three).
- Castellote J, Ariza J, Rota R, Girbau A, Xiol X. Serious drug-induced liver disease secondary to ezetimibe. World J Gastroenterol. 2008;14:5098–9. [PMC free article: PMC2742942] [PubMed: 18763297](56 year old woman developed jaundice and pruritus 2 months after starting ezetimibe without a statin or other lipid lowering medication [bilirubin 35.2 mg/dL, ALT 1575 U/L, Alk P 115 U/L], resolving within 4 weeks of stopping therapy).
- Florentin M, Liberopoulos EN, Elisaf MS. Ezetimibe-associated adverse effects: what the clinician needs to know. Int J Clin Pract. 2008;62:88–96. [PubMed: 18173814](Review of safety and toxicity of ezetimibe; rate of ALT elevations above 3 times ULN reported to be the same with ezetimibe as with placebo, but higher rates found with combinations of ezetimibe and statins [1.3% vs 0.4% with statin alone]; 4 cases of clinically apparent liver injury have appeared in the published literature).
- Ritchie SR, Orr DW, Black PN. Severe jaundice following treatment with ezetimibe. Eur J Gastroenterol Hepatol. 2008;20:572–3. [PubMed: 18467918](49 year old woman developed jaundice 4 months after starting ezetimibe [bilirubin 37.8 mg/dL but ALT and Alk P normal], with cirrhosis on liver biopsy, high bilirubin levels fell to 2.1 mg/dL 4 months later; jaundice possibly related to competition between ezetimibe and bilirubin for excretion by MRP2, the protein deficient in Dubin-Johnson syndrome).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug-Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, 3 cases were attributed to the combination of simvastatin and ezetimibe, but no details provided).
- Robinson JG, Ballantyne CM, Grundy SM, Hsueh WA, Parving HH, Rosen JB, Adewale AJ, et al. Lipid-altering efficacy and safety of ezetimibe/simvastatin versus atorvastatin in patients with hypercholesterolemia and the metabolic syndrome (from the VYMET study). Am J Cardiol. 2009;103:1694–702. [PubMed: 19539078](Controlled trial of ezetimibe/simvastatin versus atorvastatin alone in 1128 patients with metabolic syndrome and hypercholesterolemia; adverse event rates were similar in the two groups, but consecutive ALT or AST elevations above 3 times ULN occurred in 0.3% of atorvastatin vs 1.4% of ezetimibe/simvastatin treated patients).
- McGinnis B, Schimmer J, Hutka K. An evaluation of alanine transaminase and creatine kinase elevations with the use of ezetimibe in an ambulatory care setting. J Clin Lipidol. 2010;4:501–7. [PubMed: 21122697](Among 4332 patients treated with ezetimibe in an ambulatory care setting, 44 [1.1%] had an ALT elevation above 3 times ULN, but many of the 44 had other reasons for the abnormalities).
- Foody JM, Brown WV, Zieve F, Adewale AJ, Flaim D, Lowe RS, Jones-Burton C, Tershakovec AM. Safety and efficacy of ezetimibe/simvastatin combination versus atorvastatin alone in adults ≥65 years of age with hypercholesterolemia and with or at moderately high/high risk for coronary heart disease (the VYTELD study). Am J Cardiol. 2010;106:1255–63. [PubMed: 21029821](Among 1289 elderly patients with hypercholesterolemia treated with atorvastatin or the combination of ezetimibe and simvastatin for 12 weeks, adverse events were similar in the two groups, ALT values rose above 3 times ULN in 0.4% vs 0.4% of subjects and none developed clinically apparent hepatitis).
- Bays HE, Davidson MH, Massaad R, Flaim D, Lowe RS, Tershakovec AM, Jones-Burton C. Safety and efficacy of ezetimibe added on to rosuvastatin 5 or 10 mg versus up-titration of rosuvastatin in patients with hypercholesterolemia (the ACTE Study). Am J Cardiol. 2011;108:523–30. [PubMed: 21596364](Trial of adding ezetimibe to rosuvastatin vs increasing the dose in 440 patients followed for 6 weeks; only one of 221 patients on the combination vs none on the increased dose of rosuvastatin had an ALT elevation above 3 times ULN).
- Toth PP, Morrone D, Weintraub WS, Hanson ME, Lowe RS, Lin J, Shah AK, Tershakovec AM. Safety profile of statins alone or combined with ezetimibe: a pooled analysis of 27 studies including over 22,000 patients treated for 6-24 weeks. Int J Clin Pract. 2012;66:800–12. [PubMed: 22805272](Analysis of side effects from 27 controlled trials in 22,000 patients found ALT or AST elevations above 3 times ULN in 0.35% vs 0.56% of those receiving a statin alone vs its combination with ezetimibe, and no cases of clinically apparent liver injury).
- Rosen JB, Jimenez JG, Pirags V, Vides H, Hanson ME, Massaad R, McPeters G, et al. A comparison of efficacy and safety of an ezetimibe/simvastatin combination compared with other intensified lipid-lowering treatment strategies in diabetic patients with symptomatic cardiovascular disease. Diab Vasc Dis Res. 2013;10:277–86. [PubMed: 23288881](None of 321 patients who were treated with simvastatin and ezetimibe for six weeks had ALT or AST elevations above 3 times ULN).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 2 attributed to atorvastatin and 1 to simvastatin, but none to ezetimibe).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to ezetimibe or other cholesterol lowering agents).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 41 [5.4%] were attributed to lipid lowering agents including 2 to the combination of simvastatin and ezetimibe, but none to ezetimibe alone).
- Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, et al. IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97. [PubMed: 26039521](Among 18,144 patients with an acute coronary syndrome and hypercholesterolemia treated with simvastatin [40 mg daily] with or without ezetimibe for a median duration of 6 years, LDL-cholesterol levels were lower [54 vs 70 mg/dL] and atherosclerotic event rates less with addition of ezetimibe [33% vs 35%], while adverse event rates were similar, including ALT or AST elevations above 3 times ULN [2.5% vs 2.3%] and no liver related serious adverse events were mentioned).
- Kusters DM, Caceres M, Coll M, Cuffie C, Gagné C, Jacobson MS, Kwiterovich PO, et al. Efficacy and safety of ezetimibe monotherapy in children with heterozygous familial or nonfamilial hypercholesterolemia. J Pediatr. 2015;166:1377–84. [PubMed: 25841542](Among 138 children with hypercholesterolemia who were treated with ezetimibe or placebo for 12 weeks, LDL-cholesterol decreased by 27% and ezetimibe was "well tolerated", ALT elevations above 3 times ULN occurring in only 1 patient in whom values rose to 145 U/L [bilirubin and Alk P normal] and led to early discontinuation when ALT levels remained elevated for more than a month).
- Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, Bruckert E, et al. ODYSSEYALTERNATIVE Investigators. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEYALTERNATIVE randomized trial. J Clin Lipidol. 2015;9:758–69. [PubMed: 26687696](Among 313 patients with hypercholesterolemia and statin intolerance treated with alirocumab or ezetimibe or atorvastatin for 24 weeks, alirocumab produced greater reductions in LDL-cholesterol than ezetimibe with slightly lower rates of myalgia than atorvastatin [25% vs 27%] and there were no ALT elevations above 3 times ULN in any group).
- Kim KJ, Kim SH, Yoon YW, Rha SW, Hong SJ, Kwak CH, Kim W, et al. Effect of fixed-dose combinations of ezetimibe plus rosuvastatin in patients with primary hypercholesterolemia: MRS-ROZE (Multicenter Randomized Study of ROsuvastatin and eZEtimibe). Cardiovasc Ther. 2016;34:371–82. [PMC free article: PMC5108468] [PubMed: 27506635](Among 412 patients with hypercholesterolemia who were treated with rosuvastatin with vs without ezetimibe for 8 weeks, there were no serious adverse events and transient ALT elevations above 3 times ULN arose in only 1 patient in each group [0.5% vs 0.5%]).
- Jones PH, Bays HE, Chaudhari U, Pordy R, Lorenzato C, Miller K, Robinson JG. Safety of alirocumab (a PCSK9 monoclonal antibody) from 14 randomized trials. Am J Cardiol. 2016;118:1805–11. [PubMed: 27729106](Among 5234 patients treated with alirocumab or placebo or ezetimibe in 14 controlled trials, overall adverse event rates, including serious events and deaths, were similar in the three groups, although local injection reactions were more common with alirocumab; rates of ALT elevations were also similar [2.7 and 2.9% vs 2.3% with placebo and 2.6% with ezetimibe] and there were no cases of ALT elevations with jaundice that could not be attributed to other causes [2 in patients treated with alirocumab, 2 with placebo, none with ezetimibe]).
- Oikawa S, Yamashita S, Nakaya N, Sasaki J, Kono S., Effect of Fenofibrate and Ezetimibe Combination Treatment on Lipid (EFECTL) Study Investigators. Efficacy and safety of long-term coadministration of fenofibrate and ezetimibe in patients with combined hyperlipidemia: results of the EFECTL study. J Atheroscler Thromb. 2017;24:77–94. [PMC free article: PMC5225135] [PubMed: 27397061](Among 236 Japanese patients with hyperlipidemia treated with fenofibrate, ezetimibe or both for 52 weeks, ALT elevations occurred in 0% on ezetimibe, 3.8% on fenofibrate and 1.9% on both, and all changes were considered "comparatively mild").
- El Shahawy M, Cannon CP, Blom DJ, McKenney JM, Cariou B, Lecorps G, Pordy R, Chaudhari U, Colhoun HM, et al. Efficacy and safety of alirocumab versus ezetimibe over 2 years (from ODYSSEY COMBO II). Am J Cardio. 2017;120:931–9. [PubMed: 28750828](Among 720 patients with inadequate control of cholesterol levels with maximal doses of statins who were treated with alirocumab or ezetimibe for up to 2 years, adverse event rates were similar, ALT elevations above 3 times ULN occurred in 2.1% on alirocumab vs 0.8% on ezetimibe, but the abnormalities were usually a single value and no patient developed clinically apparent liver injury).
- Hong SJ, Jeong HS, Ahn JC, Cha DH, Won KH, Kim W, Cho SK, et al. A phase III, multicenter, randomized, double-blind, active comparator clinical trial to compare the efficacy and safety of combination therapy with ezetimibe and rosuvastatin versus rosuvastatin monotherapy in patients with hypercholesterolemia: I-ROSETTE (Ildong Rosuvastatin & Ezetimibe for Hypercholesterolemia) randomized controlled trial. Clin Ther. 2018;40:226–241.e4. [PubMed: 29402522](Among 396 Korean patients with hypercholesterolemia treated with rosuvastatin [5, 10 or 20 mg daily] with or without ezetimibe [10 mg daily] for 8 weeks, the percent decrease in LDL-cholesterol was higher at each dose of rosuvastatin combined with ezetimibe [overall -57% vs -44%] and the proportion of patients achieving a targeted goal of LDL-cholesterol was also higher [92% vs 80%], while there were similar rates of total adverse events [11.2% vs 11.3%], serious adverse events [0.5% vs 0.5%] as well as ALT elevations above 3 times the ULN [0.6% vs 0]).
- Lipid-lowering drugs. Med Lett Drugs Ther. 2019;61(1565):17–24. [PubMed: 30845106](Concise review of the mechanism of action, relative efficacy, safety and costs of lipid lowering drugs including statins, ezetimibe, PCSK9 inhibitors, bile acid sequestrants, fibric acid derivatives niacin and fish oil, mentions that statin therapy is associated with ALT elevations above 3 times ULN in 1-3% of patients but “whether statins actually cause liver damage is unclear”).
- Kanagalingam T, Lazarte J, Wong DKH, Hegele RA. Liver injury associated with ezetimibe monotherapy. CJC Open. 2020;3:195–197. [PMC free article: PMC7893188] [PubMed: 33644733](59 year old white man with hyperlipidemia developed anorexia, arthralgia, abdominal pain and itching 2-3 months after starting ezetimibe monotherapy [bilirubin 0.8 mg/dL, ALT 300 U/L, Alk P 1344 U/L, INR 1.0, ANA positive,] with little improvement on stopping until prednisone was added with resolution of liver abnormalities, but continued therapy with azathioprine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia.[J Atheroscler Thromb. 2007]Review Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia.Davis HR, Veltri EP. J Atheroscler Thromb. 2007 Jun; 14(3):99-108.
- Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study.[Am Heart J. 2005]Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study.Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Am Heart J. 2005 Mar; 149(3):464-73.
- Review Ezetimibe: a selective cholesterol absorption inhibitor.[Pharmacotherapy. 2003]Review Ezetimibe: a selective cholesterol absorption inhibitor.Nutescu EA, Shapiro NL. Pharmacotherapy. 2003 Nov; 23(11):1463-74.
- Ezetimibe ameliorates atherosclerotic and inflammatory markers, atherogenic lipid profiles, insulin sensitivity, and liver dysfunction in Japanese patients with hypercholesterolemia.[J Atheroscler Thromb. 2012]Ezetimibe ameliorates atherosclerotic and inflammatory markers, atherogenic lipid profiles, insulin sensitivity, and liver dysfunction in Japanese patients with hypercholesterolemia.Tamaki N, Ueno H, Morinaga Y, Shiiya T, Nakazato M. J Atheroscler Thromb. 2012; 19(6):532-8. Epub 2012 Feb 22.
- Review Pharmacology and therapeutics of ezetimibe (SCH 58235), a cholesterol-absorption inhibitor.[Clin Ther. 2003]Review Pharmacology and therapeutics of ezetimibe (SCH 58235), a cholesterol-absorption inhibitor.Jeu L, Cheng JW. Clin Ther. 2003 Sep; 25(9):2352-87.
- Ezetimibe - LiverToxEzetimibe - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...