NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ethosuximide is an succinimide based anticonvulsant commonly used for absence (petit mal) seizures in both adults and children. Ethosuximide has been associated with rare instances of serum enzyme elevations during treatment, but has not been linked to cases of clinically apparent liver injury with jaundice.
Background
Ethosuximide (eth' oh sux" a mide) is a succinimide derivative and potent anticonvulsant that has been used to treat absence (petit mal) seizures for more than 50 years. Ethosuximde reduces theshold calcium currents in thalamic neurons and suppresses the paroxysmal spike and wave activity that is associated with lapses of consciousness that occur with absence seizures. Ethosuximide was approved for use in epilepsy in 1960 for use alone or in combination with other agents to treat absence seizures. Ethosuximide is available as tablets of 250 mg and as syrup for pediatric use in several generic forms and under the brand name of Zarontin. The recommended initial dose in adults and children above the age of 6 years is 250 mg twice daily with dose escalation weekly based upon tolerance and effect. Common side effects include dizziness, somnolence, ataxia, fatigue, irritability, anorexia and epigastric discomfort. Rare, but potentially severe adverse events include hypersensitivity reactions and drug induced lupus erythematosus and scleroderma.
Hepatotoxicity
Prospective studies suggest that chronic ethosuximide therapy is not accompanied by significant elevations in serum aminotransferase levels, but can increase gamma glutamyltranspeptidase levels. Clinically apparent hepatotoxicity from ethosuximide is very rare with few case reports published despite use of this agent for half a century. Futhermore, the liver injury in reported cases was usually mild and asymptomatic and a part of a generalized hypersensitivity syndrome with fever, rash, facial edema, lymphadenopathy, and eosinophilia or atypical lymphocytosis. The usual latency to onset of the hypersensitivity syndrome is 2 to 8 weeks. The typical serum enzyme elevations are a mixed-cholestatic-hepatocellular pattern and reported cases have not been jaundiced. While the product labeling for ethosuximide warns of hepatic dysfunction and recommends periodic monitoring of liver tests, clinically apparent liver injury with jaundice from ethosuximide is rare.
Likelihood score: E* (suspected but unproven cause of clinically apparent liver injury).
Mechanism of Injury
Ethosuximide is metabolized to inactive intermediates in the liver via the cytochrome P450 system (CYP 3A4). The mechanism of ethosuximide hepatotoxicity is unknown but is likely to be hypersensitivity to an metabolic intermediate.
Outcome and Management
Reported instances of liver injury from ethosuximide have consisted of transient, asymptomatic serum enzyme elevations or mild liver injury accompanying skin rash and systemic hypersensivity reactions. Ethosuximide has not been linked to cases of chronic hepatitis, vanishing bile duct syndrome or acute liver failure. The liver injury resolves rapidly with discontinuation of ethosuximide and typically recurs rapidly with reexposure, which should be avoided. There does not appear to be cross reactivity to hypersensitivity reactions or liver injury between ethosuximide and other antiseizure medications including the aromatic anticonvulsants, but caution and careful monitoring should be used in switching to another anticonvulsant after a hypersensitivity reaction to ethosuximide.
Drug Class: Anticonvulsants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ethosuximide – Zarontin®
DRUG CLASS
Anticonvulsants
COMPLETE LABELING
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ethosuximide | 77-67-8 | C7-H11-N-O2 |
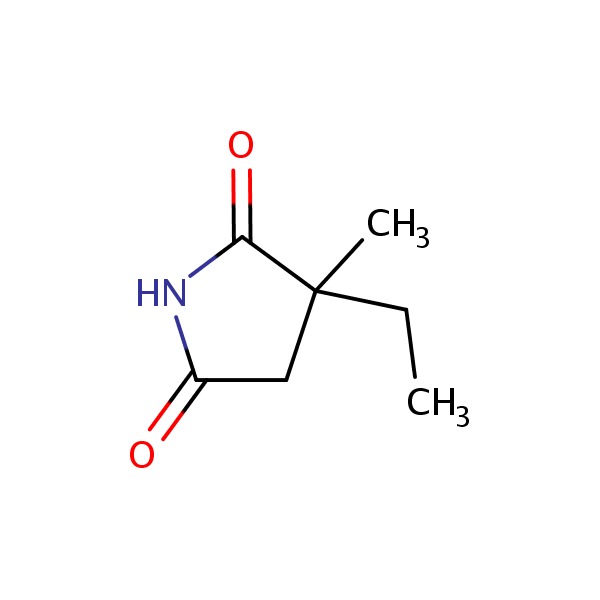 |
ANNOTATED BIBLIOGRAPHY
References updated: 19 February 2018
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999; ethosuximide is not discussed).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-442.(Review of anticonvulsant induced liver injury; ethosuximide is not discussed).
- McNamara JO. Pharmacology of the epilepsies. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 583-607.(Textbook of pharmacology and therapeutics).
- Teoh OT, Wong PK. Lupus-scleroderma syndrome induced by ethosuximide. Arch Dis Child 1975; 50: 658-61. [PMC free article: PMC1545540] [PubMed: 812426](16 year old Chinese girl with epilepsy on 4 anticonvulsants including ethosuximide for 4 years developed arthritis, sclerodactyly, rash and hepatomegaly [liver tests normal, ANA and LE prep positive], symptoms and laboratory abnormalities resolving on stopping all drugs except phenobarbital, subsequently tolerating phenytoin but having recurrence of fever and rash 3 weeks after restarting ethosuximide).
- Reynolds NC Jr, Miska RM. Safety of anticonvulsants in hepatic porphyrias. Neurology 1981; 31: 480-4. [PubMed: 7194443](Mentions that ethosuximide has been associated with triggering attacks of acute intermittent porphyria).
- Coulter DL. Ethosuximide-induced liver dysfunction. Arch Neurol 1983; 40: 393-4. [PubMed: 6847455](1 year old boy developed ALT elevations one month after ethosuximide was added to a chronic regimen of valproate and phenobarbital that continued to worsen after stopping valproate [bilirubin normal, ALT 69 rising 229 U/L, GGT 175 U/], and resolved rapidly when ethosuximide was stopped).
- Wyllie E, Wyllie R. Routine laboratory monitoring for serious adverse effects of antiepileptic medications: the controversy. Epilepsia 1991; 32 Suppl 5: S74-9. [PubMed: 1743173](Review of adverse effects of phenytoin, valproate, carbamazepine and ethosuximide with discussion of potential role of routine monitoring of laboratory results such as ALT; most information indicates that such monitoring is unlikely to be effective in avoiding the rare instance of severe idiosyncratic hepatic injury from anticonvulsants because of their rarity and rapidity of onset and the frequent, insignificant ALT elevations found; mentions aplastic anemia and leucopenia as being due to ethosuximide, but not hepatic injury).
- Conilleau V, Dompmartin A, Verneuil L, Michel M, Leroy D. Hypersensitivity syndrome due to 2 anticonvulsant drugs. Contact Dermatitis 1999; 41: 141-4. [PubMed: 10475512](6 year old boy with petit mal developed rash, fever, lymphadenopathy and eosinophilia a month after starting ethionamide and valproate [bilirubin not given, AST 70 U/L, GGT 50 U/L], redeveloping rash within a day of restarting ethosuximide, later tolerating lamotrigine and clonazepam).
- Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug Saf 1999; 21: 489– 501. [PubMed: 10612272](Review of anticonvulsant hypersensitivity syndrome: triad of fever, rash and internal organ injury occurring 1-8 weeks after exposure to anticonvulsant; liver being most common internal organ involved. Occurs in 1:1000-1:10,000 initial exposures to phenytoin, carbamazepine, phenobarbital or lamotrigine, unrelated to dose, perhaps predisposed by valproate; liver injury arises 1-4 weeks after onset of rash and ranges in severity from asymptomatic ALT elevations to icteric hepatitis to ALF; high mortality rate with jaundice; other organs include muscle, kidney, brain, heart and lung. Pseudolymphoma syndrome and serum sickness like syndrome are separate complications of anticonvulsants; role of corticosteroids uncertain; cross reactivity among the agents should be assumed; ethosuximide is not mentioned).
- Hamer HM, Morris HH. Hypersensitivity syndrome to antiepileptic drugs: a review including new anticonvulsants. Clevel Clin J Med 1999; 66: 239-45. [PubMed: 10199060](Clinical review of anticonvulsant syndrome, which occurs in 1-5/10,000 users, higher risk in African Americans and affected siblings; liver involvement common, but most cases are anicteric; other manifestations include facial edema, lymphadenopathy, bone marrow aplasia, pseudolymphoma, thyroiditis, interstitial nephritis, switching to valproate, levetiracetam or benzodiazepines is safe; ethosuximide is not mentioned).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the United States between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 10 were attributed to phenytoin, 10 to valproate and 1 to carbamazepine, but none to ethosuximide or other anticonvulsants).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scan 2008; 118: 281-90. [PubMed: 18341684](Review of all anticonvulsants; mentions that there is no known clinically significant hepatotoxicity associated with ethosuximide).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, valproate accounted for 6 cases, lamotrigine 5, phenytoin 5, gabapentin and topiramate 1 each; none were due to ethosuximide).
- Posner E. Absence seizures in children. Clin Evid (Online) 2008 Jan 10;2008. [PMC free article: PMC2907950] [PubMed: 19450342](Review of evidence of efficacy and safety of drugs for petit mal seizures in children; ethosuximide is considered effective, but there are no randomized controlled trials to support this consensus; mentions that hepatic impairment, aplastic anaemia and serious skin reactions are rare adverse effects of ethosuximide).
- Penovich PE, Willmore LJ. Use of a new antiepileptic drug or an old one as first drug for treatment of absence epilepsy. Epilepsia 2009; 50 Suppl 8:37-41. [PubMed: 19702732](Review of the therapy of absence seizures, mentions that side effects of ethosuximide "tend to be a challenge"; no mention of hepatotoxicity).
- Knowles SR, Dewhurst N, Shear NH. Anticonvulsant hypersensitivity syndrome: an update. Expert Opin Drug Saf 2012; 11: 767-78. [PubMed: 22794330](Updated review of anticonvulsant hypersensitivity syndrome; associated with phenytoin, phenobarbital, lamotrigine and carbamazepine and rarely with zonisamide, valproate and oxcarbazepine; ethosuximide not mentioned).
- Drugs for epilepsy. Treat Guidel Med Lett 2013; 11: 9-18. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants; ethosuximide is approved for treatment of absence seizures, but is not effective in generalized tonic-clonic or partial seizures; rare side effects include severe skin and generalized hypersensitivity reactions; no mention of hepatotoxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, but none were attributed to ethosuximide).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were attributed to anticonvulsants, but none specificaly to ethosuximide).
- Gironell A, Marin-Lahoz J. Ethosuximide for essential tremor: an open-label trial. Tremor Other Hyperkinet Mov (N Y) 2016; 6: 378. [PMC free article: PMC4947198] [PubMed: 27625899](A small trial of ethosuximide in 15 elderly patients with essential tremor was discontinued early because of side effects of headache, dizziness and anxiety, and lack of any effect on tremor in those able to tolerate treatment; no mention of ALT elevations).
- Drugs for epilepsy. Med Lett Drugs Ther 2017; 59 (1526): 121-30. [PubMed: 28746301](Concise review of the drugs available for therapy of epilepsy lists ethosuximide as the drug of choice for absence seizures and mentions that it is generally well tolerated, but rare adverse events can include erythema multiforme, Stevens Johnson syndrome and systemic lupus erythematosus; no mention of ALT elevations or hepatotoxicity).
- Vidaurre J, Gedela S, Yarosz S. Antiepileptic drugs and liver disease. Pediatr Neurol 2017; 77: 23-36. [PubMed: 29097018](Review of the use of anticonvulsants in patients with liver disease mentions that close monitoring is advised when using ethosuximide in patients with hepatic disfunction, but that reports of hepatoxicity of ethosuximide have been few and usually confounded by its use with other agents).
- Toyama T, Asano Y, Taniguchi T, Takahashi T, Ichimura Y, Tamaki Z, Kagami S, et al. Ethosuximide-induced lupus-scleroderma syndrome with disease-specific autoantibodies. Eur J Dermatol 2017; 27: 196-7. [PubMed: 28026802](8 year old Japanese girl with absence seizures developed fever, cold fingers, oral ulcers, arthralgias abd sclerodactyly one month after starting ethosuximide [ANA and anti-dsDNA positive, ESR elevated, "and slightly elevated transaminases"], resolving slowly with stopping).
- Tachibana K, Hamada T, Tsuchiya H, Shibata T, Fujii K, Kobayashi K, Iwatsuki K. Ethosuximide-induced Stevens-Johnson syndrome: beneficial effect of early intervention with high-dose corticosteroid therapy. J Dermatol 2018 Feb 11. [Epub ahead of print] [PubMed: 29430697](Two 4 year old children with absence seizures developed Stevens Johnson syndrome 2 and 3 weeks after starting ethosuximide [bilirubin and Alk P not provided, AST 186 and 45 U/L], resolving rapidly with immediate therapy with intravenous corticosteroids).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Methsuximide.[LiverTox: Clinical and Researc...]Review Methsuximide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- [ETHOSUXIMIDE (ZARONDAN) IN THE TREATMENT OF PETIT MAL EPILEPSY AND RELATED SEIZURES].[Ugeskr Laeger. 1964][ETHOSUXIMIDE (ZARONDAN) IN THE TREATMENT OF PETIT MAL EPILEPSY AND RELATED SEIZURES].PALUDAN J, KIORBOE E, TROLLE E, OVERVAD E. Ugeskr Laeger. 1964 Feb 13; 126:201-3.
- Epileptiform seizures in domestic fowl. IX. Implications of the absence of anticonvulsant activity of ethosuximide in a pharmacological model of epilepsy.[Can J Physiol Pharmacol. 1978]Epileptiform seizures in domestic fowl. IX. Implications of the absence of anticonvulsant activity of ethosuximide in a pharmacological model of epilepsy.Davis HL, Johnson DD, Crawford RD. Can J Physiol Pharmacol. 1978 Oct; 56(5):893-6.
- ZARONTIN (ETHOSUXIMIDE) IN THE TREATMENT OF PETIT MAL AND RELATED DISORDERS.[Epilepsia. 1964]ZARONTIN (ETHOSUXIMIDE) IN THE TREATMENT OF PETIT MAL AND RELATED DISORDERS.KIORBOE E, PALUDAN J, TROLLE E, OVERVAD E. Epilepsia. 1964 Mar; 5:83-9.
- Review Ethosuximide: from bench to bedside.[CNS Drug Rev. 2007]Review Ethosuximide: from bench to bedside.Gören MZ, Onat F. CNS Drug Rev. 2007 Summer; 13(2):224-39.
- Ethosuximide - LiverToxEthosuximide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...