NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ertapenem is a broad spectrum carbapenem antibiotic used primarily for the treatment of aerobic gram-negative bacterial infections. Ertapenem, like other carbapenems, is associated with transient and asymptomatic elevations in serum enzymes. The carbapenems have also been linked to rare instances of clinically apparent, acute cholestatic liver injury.
Background
Ertapenem (er" ta pen' em) is a broad spectrum beta-lactam antibiotic used predominantly for treatment of severe aerobic gram-negative infections. Ertapenem, like other carbapenems, binds to bacterial penicillin binding proteins and interferes with bacterial cell wall integrity and synthesis. It is a broad spectrum antibiotic with activity against many aerobic and anaerobic gram-positive and gram-negative organisms, including Staphylococcus aureus, Streptococcus pyogenes, Streptococcus agalactiae, viridans group streptococci, Enterococcus faecalis, Escherichia coli, Proteus mirabilis, Bacteroides fragilis and Peptostreptococcus species. Ertapenem was approved for use in the United States in 2001, and its use is largely restricted to serious infections in hospitalized patients. Ertapenem is indicated for the treatment of severe or complicated skin, tissue, joint, respiratory tract, intraabdominal, urinary tract and urogenital infections as well as endocarditis and sepsis due to susceptible organisms. The recommended dosage is 1 gram given intramuscularly once daily for 5 to 28 days. It is currently available as Invanz. The most common side effects of ertapenem are infusion site pain and phlebitis, diarrhea, nausea, rash, pruritus and headache.
Hepatotoxicity
Mild, transient, asymptomatic elevations in serum aminotransferase levels occur in about 5% of patients receiving parenteral ertapenem for 5 to 14 days. These abnormalities are usually self-limited and asymptomatic. In the limited period that it has been available, no cases of hepatitis with jaundice have been reported. Nevertheless, several instances of cholestatic jaundice arising during or shortly after therapy have been reported with other carbapenems. The latency to onset has been within 1 to 3 weeks and the pattern of enzyme elevations is usually cholestatic. Immunoallergic features can occur but autoantibodies are rare. The course is usually self-limiting, but at least one case of vanishing bile duct syndrome related to a carbapenem has been reported. Ertapenem and other carbapenems have not been linked to cases of acute liver failure.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury).
Mechanism of Liver Injury
The cause of the mild, transient serum enzyme elevations during ertapenem therapy is not known. The cholestatic hepatitis attributed to the carbapenems is probably immunoallergic and resembles the rare clinically apparent liver injury that has been linked to penicillins and cephalosporins.
Outcome and Management
The liver injury due to the carbapenems is usually mild, asymptomatic and self-limited. Rarely, the carbapenems can cause a clinically apparent acute cholestatic hepatitis that is usually self-limiting and not requiring therapy or intervention. There is little information on possible cross sensitivity to liver injury among the different beta-lactam antibiotics, but patients with clinically apparent liver injury due to ertapenem should probably avoid the other carbapenems.
References to the safety and potential hepatotoxicity of ertapenem are given in the Overview section on Carbapenems.
Drug Class: Antiinfective Agents, Carbapenems
Other Drugs in the Subclass, Carbapenems: Doripenem, Imipenem, Meropenem
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ertapenem – Invanz®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
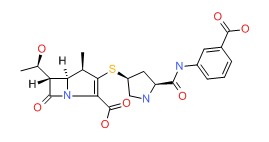
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ertapenem | 153832-46-3 | C22-H25-N3-O7-S |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Doripenem.[LiverTox: Clinical and Researc...]Review Doripenem.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Carbapenem stewardship with ertapenem and antimicrobial resistance-a scoping review.[Rev Soc Bras Med Trop. 2020]Review Carbapenem stewardship with ertapenem and antimicrobial resistance-a scoping review.Zequinão T, Telles JP, Gasparetto J, Tuon FF. Rev Soc Bras Med Trop. 2020; 53:e20200413. Epub 2020 Nov 6.
- Review Comparative review of the carbapenems.[Drugs. 2007]Review Comparative review of the carbapenems.Zhanel GG, Wiebe R, Dilay L, Thomson K, Rubinstein E, Hoban DJ, Noreddin AM, Karlowsky JA. Drugs. 2007; 67(7):1027-52.
- Review Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.[Drugs. 2022]Review Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Zhanel GG, Pozdirca M, Golden AR, Lawrence CK, Zelenitsky S, Berry L, Schweizer F, Bay D, Adam H, Zhanel MA, et al. Drugs. 2022 Apr; 82(5):533-557. Epub 2022 Mar 16.
- Review Carbapenem stewardship: does ertapenem affect Pseudomonas susceptibility to other carbapenems? A review of the evidence.[Int J Antimicrob Agents. 2012]Review Carbapenem stewardship: does ertapenem affect Pseudomonas susceptibility to other carbapenems? A review of the evidence.Nicolau DP, Carmeli Y, Crank CW, Goff DA, Graber CJ, Lima AL, Goldstein EJ. Int J Antimicrob Agents. 2012 Jan; 39(1):11-5. Epub 2011 Nov 1.
- Ertapenem - LiverToxErtapenem - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...