NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Entecavir is a guanosine nucleoside analogue used in the treatment of chronic hepatitis B virus (HBV) infection. Entecavir therapy can be associated with flares of the underlying hepatitis B during or after therapy, but has not been linked to cases of clinically apparent liver injury.
Background
Entecavir (en tek' a vir) is a guanosine analogue with potent activity against HBV and minimal activity against HIV. Entecavir is phosphorylated intracellularly where it acts by competing with guanosine for uptake by the HBV DNA polymerase and incorporation into the growing HBV DNA molecule, leading to inhibition of polymerase activity and chain termination. Entecavir lowers HBV DNA levels and leads to improvements in serum aminotransferase levels in the majority of patients. Long term therapy is well tolerated and antiviral resistance to entecavir is rare. Entecavir was approved by the FDA in 2005 and is currently widely used. Entecavir is indicated for the treatment of chronic hepatitis B and is available in 0.5 and 1 mg tablets under the brand name of Baraclude. An oral solution of 0.05 mg/mL is also available. The recommended dose of entecavir is 0.5 mg orally once daily in adults (and children over the age of 16) with typical chronic hepatitis B and 1.0 mg once daily for patients with lamivudine-resistant HBV infection. Entecavir has slight antiviral activity against HIV which predisposes to antiviral resistance and should not be used alone in patients with HBV-HIV co-infection. Side effects from entecavir are infrequent.
Hepatotoxicity
Elevations in serum ALT levels occur in 2% to 10% patients with chronic hepatitis B treated with entecavir. These elevations appear to be due to a transient flare in the underlying chronic hepatitis B and occur both during and after therapy. On treatment, ALT flares typically occur during the first 1 to 2 months of therapy and are mild, asymptomatic and self-limited, accompanying the rapid declines in HBV DNA levels. Withdrawal flares in ALT levels occur in 8% to 12% of patients who receive entecavir therapy of hepatitis B and are subsequently withdrawn from treatment. The withdrawal flares arise within 1 to 3 months of stopping treatment and are usually preceded by marked and sudden rises in HBV DNA levels towards pretreatment values. Withdrawal flares of hepatitis B can be symptomatic and severe, and several instances of acute liver failure have been described in patients withdrawn from therapy after 1 to 3 years of treatment. There have been few reports of withdrawal flares after stopping entecavir, because the majority of patients have continued therapy indefinitely. However, the rate of flares and their severity are likely to be similar after stopping entecavir as after withdrawal of other therapies of hepatitis B.
Several instances of lactic acidosis have been reported in patients with advanced hepatitis B who were treated with entecavir; however, lactate levels were reported to be normal in patients with cirrhosis started on entecavir and followed prospectively, and in large clinical trials lactic acidosis has not been reported. The cases of lactic acidosis attributed to entecavir occurred largely in patients with severe, advanced disease and may have been due to septicemia, hypotension and/or hepatic failure rather than an adverse reaction to entecavir. Therapy with entecavir has not been associated with development of the typical syndrome of lactic acidosis, hepatic steatosis and liver failure in patients without severe preexisting liver disease that has been described with stavudine, didanosine and zidovudine treatment. Thus, clinically apparent direct hepatotoxicity from entecavir must be rare, if it occurs at all.
Likelihood score: E* (suspected but unproven cause of clinically apparent liver rinjury).
Mechanism of Injury
The apparent lack of significant hepatotoxicity from entecavir may be due to its lack of hepatic metabolism. In vitro studies have shown that it does not inhibit mitochondrial function or DNA polymerase gamma.
Outcome and Management
The ALT elevations associated with initiation of entecavir use are typically asymptomatic and transient and do not warrant dose modification or discontinuation. Patients who are withdrawn from entecavir therapy should be followed carefully for evidence of a withdrawal flare and therapy reinstituted rapidly if any signs of severe liver injury arise.
Agents used in therapy of HBV infection include adefovir, emtricitabine, entecavir, lamivudine, telbivudine, tenofovir, interferon alfa and peginterferon.
Drug Class: Antiviral Agents, Antiretroviral Agents, Hepatitis B Agents
Other Drugs in the Subclass, Nucleoside Analogues: Abacavir, Adefovir, Didanosine, Emtricitabine, Lamivudine, Stavudine, Telbivudine, Tenofovir, Zidovudine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Entecavir – Baraclude®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
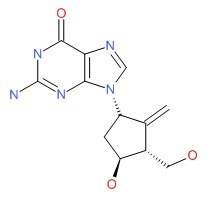
| Entecavir | 142217-69-4 | C12-H15-N5-O3 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 10 February 2018
- Núñez M. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 505-518.(Review of hepatotoxicity of antiviral agents including agents for hepatitis B).
- Acosta EP, Flexner C. Antiviral agents (nonretroviral). In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1593-1622.(Textbook of pharmacology and therapeutics).
- Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, Boron-Kaczmarska A, et al.; AI463026 BEHoLD Study Group. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology 2006; 130: 2039-49. [PubMed: 16762627](Trial comparing entecavir [n=141] to lamivudine [n=145] for lamivudine resistant chronic hepatitis B; ALT flares [>10 times normal] occurred in <1% of entecavir [initial on treatment flare] vs 11% of lamivudine-recipients).
- Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, et al.; BEHoLD AI463022 Study Group. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006; 354: 1001-10. [PubMed: 16525137](Controlled trial comparing entecavir [n=354] to lamivudine [n=355] in patients with HBeAg-positive chronic hepatitis B; on-treatment ALT elevations >5 times ULN occurred in 10% of entecavir- vs 17% of lamivudine-treated subjects [entecavir therapy stopped in one patient]; posttreatment elevations in 2% vs 12%).
- Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, et al.; BEHoLD AI463027 Study Group. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006; 354: 1011-20. [PubMed: 16525138](Controlled trial comparing entecavir [n=325] to lamivudine [n=313] in patients with HBeAg-negative chronic hepatitis B; on-treatment ALT elevations >5 times normal occurred in 2% of entecavir- vs 3% of lamivudine-treated; posttreatment elevations in 12% vs 29%, no deaths from flares).
- Mazzucco CE, Hamatake RK, Colonno RJ, Tenney DJ. Entecavir for treatment of hepatitis B virus displays no in vitro mitochondrial toxicity or DNA polymerase gamma inhibition. Antimicrob Agents Chemother 2008; 52: 598-605. [PMC free article: PMC2224751] [PubMed: 18056280](In vitro assay for mitochondrial toxicity found no evidence of toxicity of entecavir at 100-fold the typical maximum concentration; zalcitabine [ddC] had marked inhibitory activity).
- Schiff E, Simsek H, Lee WM, Chao YC, Sette H Jr, Janssen HL, Han SH, et al. Efficacy and safety of entecavir in patients with chronic hepatitis B and advanced hepatic fibrosis or cirrhosis. Am J Gastroenterol 2008; 103: 2776-83. [PubMed: 18721244](Analysis of 245 patients with advanced chronic hepatitis B in 3 clinical trials; on-treatment flares [ALT >10 times normal] occurred in 1 of 120 entecavir- vs 4 of 125 lamivudine treated patients).
- Sherman M, Yurdaydin C, Simsek H, Silva M, Liaw YF, Rustgi VK, Sette H, et al.; AI463026 Benefits of Entecavir for Hepatitis B Liver Disease(BEHoLD) Study Group. Entecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeks. Hepatology 2008; 48: 99-108. [PubMed: 18537189](Trial comparing entecavir [n=141] to lamivudine [n=145] for lamivudine resistant chronic hepatitis B; as published previously, ALT flares [>10 times normal] occurred in <1% of entecavir [initial on treatment flare] vs 11% of lamivudine recipients. On stopping therapy, 6 of 54 [11%] entecavir treated subjects had a withdrawal flare, but no follow up given).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 8 were attributed to antiviral agents, but none to drugs used in therapy of hepatitis B).
- Sulkowski MS. Management of hepatic complications in HIV-infected persons. J Infect Dis 2008; 197 Suppl 3: S279-93. [PubMed: 18447614](Review of the complications, natural history and management of hepatitis B in patients with HIV infection).
- Nüesch R, Ananworanich J, Srasuebkul P, Chetchotisakd P, Prasithsirikul W, Klinbuayam W, Mahanontharit A, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS 2008; 22: 152-4. [PubMed: 18090405](Among 10 patients with HIV-HBV coinfection on tenofovir therapy, 6 had a rise in HBV DNA levels and one had a clinically significant flare of hepatitis within 6 months of tenofovir withdrawal; all responded to restarting).
- Sugiura K, Sugiura M, Takashi T, Naoki H, Itoh A. Immediate allergy, drug-induced eruption, by Entecavir. J Eur Acad Dermatol Venereol 2009; 23:487-9. [PubMed: 18721215](30 year old man with chronic hepatitis B developed a rash within 2 days of starting entecavir and had a positive skin test reaction; tolerated being switched to lamivudine without recurrence; role of entecavir uncertain as ALT was 3219 U/L on day it was started and had fallen to 921 U/L by 4 days).
- Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 2009; 49: 1503-14. [PubMed: 19280622](Analysis of 663 patients from 6 trials of entecavir monitored for up to 5 years; antiviral resistance developed in only 1.2% of treatment-naïve, but 51% of lamivudine-resistant patients; no information on clinical or ALT features).
- Shouval D, Lai CL, Chang TT, Cheinquer H, Martin P, Carosi G, Han S, et al. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case for continuous antiviral therapy. J Hepatol 2009; 50: 289-95. [PubMed: 19070393](In a trial in 275 entecavir- vs 245 lamivudine-treated patients, 1 patient developed entecavir resistance, but no accompanying clinical information; mild on-treatment ALT flares occurred in 3 [2%] and more marked withdrawal flares in 24 [8%] of entecavir-treated patients, usually with rise in HBV DNA levels and none being clinically apparent or associated with jaundice).
- Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology 2009; 49 (5 Suppl): S185-95. [PubMed: 19399802](Review of side effects of nucleoside analogues used to treat chronic hepatitis B).
- Cohen SM, Levy RM, Jovanovich JF, Ahn J. Fatal lactic acidosis associated with the use of combination oral medications to treat reactivation of hepatitis B. J Clin Gastroenterol 2009; 43: 1008-10. [PubMed: 19461528](54 year old man with leukemia developed severe hepatitis B after a 4th monthly cycle of chemotherapy [ALT 2853 U/L, Alk P 112 U/L, bilirubin 20.9 mg/dL, INR 1.8] developed fatal lactic acidosis [pH 6.95, INR 4.4], with progressive liver failure 10 days later having started entecavir, adefovir and prednisone).
- Lange CM, Bojunga J, Hofmann WP, Wunder K, Mihm U, Zeuzem S, Sarrazin C. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology 2009; 50: 2001-6. [PubMed: 19937695](Among 16 patients with chronic hepatitis B and severe liver dysfunction started on entecavir, 5 developed evidence of lactic acidosis 4-240 days after starting, occurring only in those with severe dysfunction and accompanied by acidosis in 3).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, lamivudine [26th, 45 cases] was among the 40 most commonly implicated drugs).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 4 to antiretroviral agents, but none to agents used in the treatment of hepatitis B).
- Ortu F, Weimer LE, Floridia M, Manconi PE. Raltegravir, tenofovir, and emtricitabine in an HIV-infected patient with HCV chronic hepatitis, NNRTI intolerance and protease inhibitors-induced severe liver toxicity. Eur J Med Res 2010; 15: 81-3. [PMC free article: PMC3352050] [PubMed: 20452889](43 year old woman with HIV/HCV coinfection who developed symptomatic elevations of serum enzymes on saquinavir, fosamprenavir and again on darunavir, was adequately maintained on tenofovir/emtricitabine and raltegravir).
- Dore GJ, Soriano V, Rockstroh J, Kupfer B, Tedaldi E, Peters L, Neuhaus J, Puoti M, Klein MB, Mocroft A, Clotet B, Lundgren JD; SMART INSIGHT study group. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS 2010; 24: 857-65. [PMC free article: PMC2881334] [PubMed: 20216301](Among 54 patients with HIV-HBV coinfection who were withdrawn from chronic antiretroviral therapy, 12 [22%] had a >3 log rebound in HBV DNA levels, including 7 of 17 [41%] who had been on tenofovir vs 3 of 27 [11%] on lamivudine; ALT flares were uncommon and no patient developed hepatic decompensation).
- Petersen J, Ratziu V, Buti M, Janssen HL, Brown A, Lampertico P, Schollmeyer J, et al. Entecavir plus tenofovir combination as rescue therapy in pre-treated chronic hepatitis B patients: An international multicenter cohort study. J Hepatol 2012; 56: 520-6. [PubMed: 22037226](Among 57 patients with chronic hepatitis B and advanced liver disease who had only a partial response to other antiviral agents, the combination of entecavir and tenofovir led to improvement in most patients and none developed lactic acidosis).
- Perrillo R, Buti M, Durand F, Charlton M, Gadano A, Cantisani G, Loong CC, et al. Entecavir and HBIg in patients receiving liver transplant for chronic hepatitis B. Liver Transpl 2013; 19: 887-95. [PMC free article: PMC3791551] [PubMed: 23788462](Among 65 patients with chronic hepatitis B undergoing liver transplantation who received entecavir with or without HBIG, ALT flares occured in 6 patients [9%], all within 2 weeks of transplant and not associated with an increase in HBV DNA; by 72 weeks after transplant, HBsAg returned in 2 patients [3%], both without detectable HBV DNA).
- Yu W, Zhao C, Shen C, Wang Y, Lu H, Fan J. The efficacy and safety of nucleos(t)ide analogues in patients with spontaneous acute exacerbation of chronic hepatitis B: a systematic review and meta-analysis. PLoS One 2013; 8: e65952. [PMC free article: PMC3679018] [PubMed: 23776577](Systematic review identified 15 studies of therapy of spontaneous acute exacerbations of chronic hepatitis; lamivudine and entecavir therapy were "relatively safe and well tolerated", but had no impact on short term mortality).
- Yu S, Jianqin H, Wei W, Jianrong H, Yida Y, Jifang S, Liang Y, et al. The efficacy and safety of nucleos(t)ide analogues in the treatment of HBV-related acute-on-chronic liver failure: a meta-analysis. Ann Hepatol 2013; 12: 364-72. [PubMed: 23619252](Systematic review identified 5 studies of therapy of HBV related acute-on-chronic liver failure; lamivudine and entecavir therapy were associated with reduction in short term mortality).
- Lok AS, Trinh H, Carosi G, Akarca US, Gadano A, Habersetzer F, Sievert W, et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology 2012; 143: 619-28. [PubMed: 22643350](Among 379 patients with chronic hepatitis B treated with entecavir alone or entecavir with tenofovir for 2 years, 3 had ALT flares [>10 times ULN and twice baseline] on therapy, all with associated with a decrease in HBV DNA levels and subsequent loss of HBeAg, and none of 50 patients who were withdrawn from therapy had an ALT flare).
- Manns MP, Akarca US, Chang TT, Sievert W, Yoon SK, Tsai N, Min A, Pangerl A, et al. Long-term safety and tolerability of entecavir in patients with chronic hepatitis B in the rollover study ETV-901. Expert Opin Drug Saf 2012; 11: 361-8. [PubMed: 22233350](Among 1051 patients with chronic hepatitis B treated with entecavir in a long term extension of previous controlled trials, 32 [3%] had an on-treament ALT flare [>10 times ULN and twice baseline], usually in association with a decrease in HBV DNA levels; 2 patients developed virologic breakthough and had an ALT flare when entecavir was stopped).
- Hou JL, Jia JD, Wei L, Zhao W, Wang YM, Cheng M, Tang X, et al. Efficacy and safety of entecavir treatment in a heterogeneous CHB population from a ‘real-world’ clinical practice setting in China. J Viral Hepat 2013; 20: 811-20. [PubMed: 23876210](Among 2600 real world Chinese patients with chronic hepatitis B treated with entecavir for at least one year, 2 had ALT flares on treatment, both within 2 months of starting and resolving without complications, another patient developed an acute relapse after stopping therapy and another had a serious ALT elevation; few details given).
- Luo J, Li X, Wu Y, Lin G, Pang Y, Zhang X, Ao Y, et al. Efficacy of entecavir treatment for up to 5 years in nucleos(t)ide-naïve chronic hepatitis B patients in real life. Int J Med Sci 2013; 10: 427-33. [PMC free article: PMC3590603] [PubMed: 23471472](Among 230 real life Chinese patients with chronic hepatitis B treated with entecavir for up to 5 years, none had a serious adverse event; no information provided on ALT flares of hepatotoxicity).
- Wang X, Zhang C, Zhu Y, Xiong Y, Wang Y. Efficacy of 2 years of entecavir plus adefovir therapy in patients with chronic hepatitis B who had failed on prior nucleos(t)ide analog treatment. Antiviral Res 2014; 103: 71-7. [PubMed: 24462954](Among 104 previously treated patients with chronic hepatitis B given the combination of entecavir and adefovir for at least 2 years, none developed worsening liver disease, liver decompensation of hepatocellular carcinoma).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none were attributed to agents used to treat hepatitis B).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 were attributed to antiviral agents, but none to entecavir or other drugs used to treat hepatitis B).
- Ahn J, Lee HM, Lim JK, Pan CQ, Nguyen MH, Ray Kim W, Mannalithara A, et al. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US - the ENUMERATE study. Aliment Pharmacol Ther 2016; 43: 134-44. [PMC free article: PMC4926997] [PubMed: 26510638](In a multicenter observational study of 841 patients with chronic hepatitis B treated in clinical practice, response rates were similar to those in controlled trials and serious adverse events were largely judged to be unrelated, although 8 patients discontinued treatment because of an adverse event including 2 cases of non-fatal lactic acidosis that resolved without therapy; other adverse events included rash, arthralgia, abdominal discomfort, fatigue and alopecia).
- Jonas MM, Chang MH, Sokal E, Schwarz KB, Kelly D, Kim KM, Ling SC, et al. Randomized, controlled trial of entecavir versus placebo in children with hepatitis B envelope antigen-positive chronic hepatitis B. Hepatology 2016; 63: 377-87. [PubMed: 26223345](Among 180 children or adolescents with chronic HBeAg-positive hepatitis B treated with entecavir or placebo for 48 weeks, loss of HBeAg was more frequent with entecavir [24% vs 11%], while ALT elevations above 5 times ULN were less frequent [2% vs 12%] and both instances on entecavir occurred in conjunction with loss of HBeAg).
- Lee KS, Kweon YO, Um SH, Kim BH, Lim YS, Paik SW, Heo J, et al. Efficacy and safety of entecavir versus lamivudine over 5 years of treatment: A randomized controlled trial in Korean patients with hepatitis B e antigen-negative chronic hepatitis B. Clin Mol Hepatol 2017; 23: 331-9. [PMC free article: PMC5760004] [PubMed: 28946736](Among 120 Korean patients with chronic HBeAg-negative hepatitis B treated with entecavir or lamivudine for up to 5 years, virological responses were greater with entecavir and ALT elevations above 5 times ULN occurred in 10% of those on lamivudine, but in none of those on entecavir).
- Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol 2017; 9: 227-41. [PMC free article: PMC5316843] [PubMed: 28261380](Review of the safety and adverse effects of oral antiviral nucleoside analogues [including entecavir], mentions that the product labels all have a boxed warning regarding lactic acidosis and hepatic failure, although this has not been convincingly documented to occur with the nucleoside analogues approved as therapy of hepatitis B).
- Kim CW, Kim CS, Kim HY, Lee CD, Yu K, Llamoso C, Lee HJ. Large-scale surveillance study of the safety and effectiveness of entecavir in Korean patients with chronic hepatitis B. Korean J Intern Med 2018; 33: 91-101. [PMC free article: PMC5768541] [PubMed: 29228519](Among 3366 Koreans with chronic hepatitis B treated with entecavir in routine clinical practice between 2006 and 2012, the reported adverse event rate was 7.6% and serious adverse event rate 0.3% [9 patients], all of which were considered unrelated to entecavir and there were no instances of hypersensitivity reactions, anaphylaxis or acute liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Entecavir: a new nucleoside analogue for the treatment of chronic hepatitis B.[Drugs Today (Barc). 2007]Review Entecavir: a new nucleoside analogue for the treatment of chronic hepatitis B.Rivkin A. Drugs Today (Barc). 2007 Apr; 43(4):201-20.
- Review Entecavir: a review of its use in the treatment of chronic hepatitis B in patients with decompensated liver disease.[Drugs. 2011]Review Entecavir: a review of its use in the treatment of chronic hepatitis B in patients with decompensated liver disease.Keating GM. Drugs. 2011 Dec 24; 71(18):2511-29.
- Evaluation of the cost-effectiveness of entecavir versus lamivudine in hepatitis BeAg-positive chronic hepatitis B patients.[J Manag Care Pharm. 2008]Evaluation of the cost-effectiveness of entecavir versus lamivudine in hepatitis BeAg-positive chronic hepatitis B patients.Yuan Y, Iloeje UH, Hay J, Saab S. J Manag Care Pharm. 2008 Jan-Feb; 14(1):21-33.
- Review Entecavir: a review of its use in chronic hepatitis B.[Drugs. 2009]Review Entecavir: a review of its use in chronic hepatitis B.Scott LJ, Keating GM. Drugs. 2009 May 29; 69(8):1003-33.
- A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B.[N Engl J Med. 2006]A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, et al. N Engl J Med. 2006 Mar 9; 354(10):1001-10.
- Entecavir - LiverToxEntecavir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...