NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Emtricitabine is a nucleoside analogue and reverse transcriptase inhibitor used in combination with other agents for treatment and prevention of human immunodeficiency virus (HIV) infection and the acquired immunodeficiency syndrome (AIDS). Emtricitabine does not appear to be a significant cause of drug induced liver injury, but may cause flares of disease in patients with underlying chronic hepatitis B virus (HBV) infection.
Background
Emtricitabine (em" trye sye' ta been) is an L-enantiomer and substituted analogue of cytosine (5-fluorothiocytidine: FTC) and is active against both HIV and HBV, being similar in structure and activity to lamivudine. Emtricitabine is intracellularly phosphorylated to emtricitabine 5’-triphosphate which competes with the naturally occurring deoxycytidine 5’-triphosphate for incorporation into HIV DNA by the HIV reverse transcriptase, resulting in chain termination and inhibition of the polymerase activity. Emtricitabine was approved for use in HIV infection in the United States in 2006. Current indications include treatment of HIV infection, the prophylaxis of HIV infection in cases of occupational exposure, nonoccupational exposure, and perinatal transmission. Emtricitabine is also active against HBV, but has not been specifically approved for use in hepatitis B. The combination of emtricitabine with tenofovir is used in many current antiretroviral regimens and is considered the therapy of choice in patients with HBV-HIV coinfection. Emtricitabine is available as 200 mg capsules and in an oral solution as a single agent under the brand name of Emtriva; in 200 mg tablets in combination with tenofovir disoproxil fumarate (300 mg) as Truvada; in tablets in combination with tenofovir (300 mg) and efavirenz (600 mg) as Atripla; and in capsules in combination with tenofovir (300 mg), elvitegravir (150 mg) and cobicistat (150 mg) as Stribild. The recommended dose of emtricitabine in adults is 200 mg orally once daily. The combination formulations of Truvada, Atripla and Stribild are also given orally once daily. Side effects of attributable to emtricitabine are uncommon.
Hepatotoxicity
There is little evidence for direct hepatotoxicity of emtricitabine and it has not been specifically implicated in cases of lactic acidosis with steatosis and hepatic failure. However, patients with chronic hepatitis B can experience a flare of the underlying hepatitis during emtricitabine therapy. These flares occur either at the start therapy (treatment flares), with the development of antiviral resistance (breakthrough flares), or when therapy is abruptly stopped (withdrawal flares). Treatment flares occur in 5% to 10% of patients, are usually transient and asymptomatic, and rarely require dose modification or discontinuation of therapy. In contrast, withdrawal flares occur in 15% to 30% of patients, but can be symptomatic and severe, in rare instances (~1%) leading to acute liver failure, death or requirement for emergency liver transplantation. Patients who develop emtricitabine resistance often have relapse of disease activity after the appearance of the mutant HBV strain and rise in HBV DNA levels; this relapse can initially be severe and associated with symptoms and jaundice.
Mechanism of Injury
The apparent absence of significant hepatotoxicity from emtricitabine may be due to its minimal hepatic metabolism (13%) and the fact that it is both an L-enantiomer of cytidine and is blocked at the 3’ position on the deoxyribose component, making it unlikely that emtricitabine would be used by host nuclear or mitochondrial polymerases. The flares of hepatitis B that occur with initiation, antiviral resistance or withdrawal of therapy probably represent activation of immune responses to HBV caused by the sudden change in levels of viral replication.
Outcome and Management
ALT elevations have not been associated with emtricitabine use in patients without hepatitis B. Patients with HBV infection who have a flare of disease during emtricitabine can usually be monitored carefully and continued on therapy. Patients with a flare of hepatitis due to development of antiviral resistance should be switched to or have the addition of another agent with a different profile of resistance. Patients with a withdrawal flare of hepatitis B should be evaluated rapidly and restarted on antiviral therapy, if appropriate. Cases of acute liver failure requiring liver transplantation have been reported in patients with hepatitis B withdrawn from emtricitabine therapy. Due to the correlation between HIV/HBV coinfection and liver dysfunction in a subset of patients who discontinue emtricitabine, all patients with HIV should be tested for HBV before starting therapy with emtricitabine.
Agents used in therapy of HBV infection include adefovir, emtricitabine, entecavir, lamivudine, telbivudine, tenofovir, interferon alfa and peginterferon.
Drug Class: Antiviral Agents, Antiretroviral Agents, Hepatitis B Agents
Other Drugs in the Subclass, Nucleoside Analogues: Abacavir, Adefovir, Didanosine, Entecavir, Lamivudine, Stavudine, Telbivudine, Tenofovir, Zidovudine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Emtricitabine – Emtriva®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
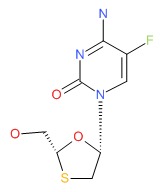
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Emtricitabine | 143491-57-0 | C8-H10-F-N3-O3-S |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 10 February 2018
- Núnez M. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 505-518.(Review of hepatotoxicity of antiviral agents).
- Flexner C. Antiretroviral agents and treatment of HIV infection. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1623-64.(Textbook of pharmacology and therapeutics).
- Acosta EP, Flexner C. Antiviral agents(nonretroviral). In, In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1593-1622.(Textbook of pharmacology and therapeutics).
- http://aidsinfo
.nih.gov/guidelines/ (Regularly updated guidelines for the use of antiretroviral agents in HIV-1 infected adults, adolescents and children). - Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nature Med 1995; 1: 417-23. [PubMed: 7585087](Review of mechanisms for mitochondrial injury by nucleoside analogues including inhibition of mitochondrial DNA polymerase gamma).
- Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JAM, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as a common pathway. AIDS 1998; 12: 1735-44. [PubMed: 9792373](Review of mitochondrial function and role of mitochondrial toxicity or depletion in the adverse side effects of nucleoside analogues).
- Kontorinis N, Dieterich D. Hepatotoxicity of antiretroviral therapy. AIDS Rev 2003; 5: 36-43. [PubMed: 12875106](Review of hepatotoxicity of antiretroviral drugs; definition of hepatotoxicity in antiretroviral studies; grade 1=1.25-2.5 times, grade 2=2.6-5 times, grade 3=5.1-10 times and grade 4=>10 times ULNl or baseline ALT values; abacavir and lamivudine [similar to emtricitabine] have been least commonly linked to hepatotoxicity).
- Gish RG, Trinh H, Leung N, Chan FK, Fried MW, Wright TL, Wang C, et al. Safety and antiviral activity of emtricitabine(FTC) for the treatment of chronic hepatitis B infection: a two-year study. J Hepatol 2005; 43: 60-66. [PubMed: 15922478](Study of 3 doses of emtricitabine given for 2 years in 98 patients with chronic hepatitis B; on-treatment ALT flares [>20 fold-normal] occurred in 6%, withdrawal flares in 19%, but no case of clinical decompensation).
- Pozniak AL, Gallant JE, DeJesus E, Arribas JR, Gazzard B, Campo RE, Chen SS, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes.a 96-week analysis. J Acquir Immune Defic Syndr 2006; 43: 535-40. [PubMed: 17057609](Controlled trial of tenofovir and emtricitabine vs zidovudine and lamivudine combined with efavirenz in 517 patients with HIV infection; elevations in ALT >3 times normal occurred in 8% vs 9% in first 2 years of study).
- Lim SG, Ng TM, Kung N, Volfova M, Husa P, Lee SS, Chan S, et al.; Emtricitabine FTCB-301 Study Group. A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B. Arch Intern Med 2006; 166: 49-56. [PubMed: 16401810](Controlled trial of emtricitabine vs placebo in 248 patients with chronic hepatitis B; therapy was stopped after 48 weeks in 145 patients, of whom 33 [23%] had a biochemical flare of disease and 1 developed acute liver failure and required emergency liver transplantation).
- Lim SG, Krastev Z, Ng TM, Mechkov G, Kotzev IA, Chan S, Mondou E, et al. Randomized, double-blind study of emtricitabine (FTC) plus clevudine versus FTC alone in treatment of chronic hepatitis B. Antimicrob Agents Chemother 2006; 50: 1642-8. [PMC free article: PMC1472200] [PubMed: 16641430](Among 81 patients with a 24 week course of emtricitabine, 16% had ALT elevations above 3 times normal on treatment and 15% had a withdrawal flare, one of which resulted in clinical decompensation).
- Jain MK. Drug-induced liver injury associated with HIV medications. Clin Liver Dis 2007; 11: 615-39, vii-viii. [PubMed: 17723923](Review of hepatotoxicity of antiretroviral medications; ALT elevations occur in 2-18% of patients, but often resolve spontaneously even without dose modification; classes of injury include hypersensitivity [nevirapine, efavirenz, abacavir], mitochondrial injury [stavudine, didanosine, zidovudine], flares of hepatitis B [lamivudine, emtricitabine, tenofovir], flares of hepatitis C [any potent regimen], idiosyncratic injury [ritonavir, nevirapine, efavirenz], cholestatic hepatitis [many agents]).
- Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, et al.; International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA 2008; 300: 555-70. [PubMed: 18677028](Updated recommendations on use of antiviral therapy in adults with HIV infection including use of recently approved agents raltegravir, maraviroc and etravirine).
- Inductivo-Yu I, Bonacini M. Highly active antiretroviral therapy-induced liver injury. Current Drug Safety 2008; 3: 4-13. [PubMed: 18690975](Review of drug induced liver injury due to antiretroviral agents; no discussion of emtricitabine).
- Soriano V, Puoti M, Garcia-Gascó Rockstroh JK, Benhamou Y, Barreiro P, McGovern B. Antiretroviral drugs and liver injury. AIDS 2008; 22: 1-13. [PubMed: 18090386](Review of hepatotoxicity of antiretroviral drugs with recommendations on management, stopping therapy if symptoms arise, with overt jaundice [direct bilirubin], evidence of mitochondrial toxicity, ALT >10 times ULN, ALT at lower levels if newly marketed agent; important to rule out other causes; problematic agents include didanosine, stavudine and zidovudine, nevirapine and efavirenz, full dose ritonavir and tipranavir).
- Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology 2009; 49 (5 Suppl): S185-95. [PubMed: 19399802](Review of side effects of nucleoside analogues used to treat chronic hepatitis B).
- Cohen C, Elion R, Ruane P, Shamblaw D, DeJesus E, Rashbaum B, Chuck SL, et al. Randomized, phase 2 evaluation of two single-tablet regimens elvitegravir/cobicistat/ emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for the initial treatment of HIV infection. AIDS 2011; 25: F7-12. [PubMed: 21412057](Controlled trial of at least 48 weeks of elvitegravir with cobicistat versus efavirenz, both combined with emtricitabine and tenofovir in 71 treatment naive adults with HIV infection; found similar efficacy and safety; no mention of ALT elevations or hepatotoxicity).
- Molina JM, Lamarca A, Andrade-Villanueva J, Clotet B, Clumeck N, Liu YP, Zhong L, et al.; Study 145 Team. Efficacy and safety of once daily elvitegravir versus twice daily raltegravir in treatment-experienced patients with HIV-1 receiving a ritonavir-boosted protease inhibitor: randomised, double-blind, phase 3, non-inferiority study. Lancet Infect Dis 2012; 12: 27-35. [PubMed: 22015077](Controlled trial of elvitegravir vs raltegravir, both combined with a ritonavir boosted protease inhibitor and a second antiretroviral agent in 702 treatment experienced patients with HIV infection; found similar efficacy and safety; 2 patients on elvitegravir and 5 on raltegravir stopped therapy because of acute hepatitis, but details were not given; ALT elevations above 5 times the ULN occurred in 2% of patients on elvitegravir versus 5% on raltegravir).
- Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, Gallant JE, et al.; GS-US-236-0102 study team. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379 (9835): 2439-48. [PubMed: 22748591](Controlled trial of at least 48 weeks of elvitegravir/cobicistat vs efavirenz, combined with emtricitabine and tenofovir in 700 treatment naive patients with HIV infection; found similar efficacy and safety; side effects included diarrhea, nausea, fatigue, dizziness, headache and rashes leading to discontinuation in 4% of patients; ALT elevations occurred in 15% of elvitegravir treated subjects; no mention of clinically apparent liver injury).
- DeJesus E, Rockstroh JK, Henry K, Molina JM, Gathe J, Ramanathan S, Wei X, et al.; GS-236-0103 Study Team. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet 2012; 379 (9835): 2429-38. [PubMed: 22748590](Controlled trial of at least 48 weeks of elvitegravir/cobicistat versus atazanavir /ritonavir, combined with emtricitabine and tenofovir in 708 treatment naive patients with HIV infection; found similar efficacy and safety; ALT elevations occurred in 15% of elvitegravir treated patients, but "patients with clinically significant liver function test abnormalities generally had concurrent underlying hepatic disease").
- A 4-drug combination (Stribild) for HIV. Med Lett Drugs Ther 2012; 54 (1404): 95-6. [PubMed: 23183388](Concise review of the efficacy and safety of Stribild, shortly after its approval in the US; does not mention ALT elevations or hepatotoxicity).
- Zolopa A, Sax PE, DeJesus E, Mills A, Cohen C, Wohl D, Gallant JE, et al.; GS-US-236-0102 Study Team. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/ emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr 2013; 63: 96-100. [PubMed: 23392460](Analysis of the second year of study described by Sax et al. [2012] found no new safety concerns; no mention of ALT elevations or hepatotoxicity).
- Rockstroh JK, DeJesus E, Henry K, Molina JM, Gathe J, Ramanathan S, Wei X, et al; GS-236-0103 Study Team. A randomized, double-blind comparison of coformulated Evitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr 2013; 62: 483-6. [PubMed: 23337366](Analysis of the second year of study described by DeJesus et al. [2012] found no new safety concerns; no mention of ALT elevations or hepatotoxicity).
- Drugs for HIV infection. Treat Guidel Med Lett 2014; 12 (138): 7-16. [PubMed: 24457549](Concise review of drugs for HIV infection discusses emtricitabine as "the preferred NRTI backbone for treatment-naive patients"; list of adverse events mentions hyperpigmentation of palms and soles, but does not mention ALT elevations or hepatotoxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 5 of which were attributed to antiretroviral agents, including lamivudine, zidovudine and nevirapine, but not emtricitabine or tenofovir).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 [1.3%] were attributed to antiretroviral medications, but no case was linked specifically to emtricitabine or tenofovir).
- Emtricitabine/tenofovir alafenamide (Descovy) for HIV. Med Lett Drugs Ther 2016; 58 (1500): 100-1. [PubMed: 27466750](Concise review of the mechanism of action, clinical efficacy, safety and cost of the combination of emtricitabine and tenofovir alafenamide, mentions the possibility of flares of hepatitis B when it is withdrawn).
- Odefsey--another NNRTI combination for HIV. Med Lett Drugs Ther 2016; 58 (1494): 60-1. [PubMed: 27148923](Concise review of the mechanism of action, clinical efficacy, safety and cost of the combination of emtricitabine, tenofovir alafenamide and rilpivirine; no mention of ALT elevations or hepatotoxicity).
- Genvoya--a new 4-drug combination for HIV. Med Lett Drugs Ther 2016; 58 (1488): 19-21. [PubMed: 26859659](Concise review of the mechanism of action, clinical efficacy, safety and costs of Genvoya, a single tablet regimen of elvitegravir, tenofovir alafenamide, emtricitabine and cobicistat as therapy of HIV infection, mentions the advantage of tenofovir alafenamide in having less renal and bone toxicity than the disoproxil fumarate and gives the standard warnings about immune reconstitution syndrome with potential liver injury on starting, and reactivation of hepatitis B on stopping antiretroviral reigimens).
- Squillace N, Ricci E, Quirino T, Gori A, Bandera A, Carenzi L, De Socio GV, et al.; CISAI Study Group. Safety and tolerability of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate in a real life setting: data from surveillance cohort long-term toxicity antiretrovirals/antivirals (SCOLTA) project. PLoS One 2017; 12 (6): e0179254. [PMC free article: PMC5478131] [PubMed: 28632758](Among 280 patients with HIV infection started on therapy with elvitegravir/cobicistat, emtricitabine and tenofovir and monitored for a median of 11 months, serum enzyme elevations occurred in 23 subjects [8%], but most were less than 5 times the upper limit or normal and only 3 led to treatment interruptions).
- Zash R, Jacobson DL, Diseko M, Mayondi G, Mmalane M, Essex M, Petlo C, et al. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr 2017; 171: e172222. [PMC free article: PMC5726309] [PubMed: 28783807](Prospective surveillance of maternity hospitals in Botswana and 47,027 live births found that adverse outcomes were more common among HIV-exposed than unexposed infants [40% vs 29%], and rates were lower in those whose mothers were taking emtricitabine and tenofovir based- vs lamivudine [47%] or zidovudine [45%] based-regimens, and lower with efavirenz vs nevirapine containing regimens [36% vs 42%] and were lowest of all in those on these regimens since the time of conception [12% and 18%]).
- Orkin C, Molina JM, Negredo E, Arribas JR, Gathe J, Eron JJ, Van Landuyt E,et al.; EMERALD study group. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV 2018; 5: e23-e34. [PubMed: 28993180](Among 1141 treatment experienced adults with HIV infection who were maintained on their regular regimen or switched to a single-table formulation of darunavir, cobicistat, emtricitabine and tenofovor alafenamide and followed for 48 weeks, virologic success was similar in the two groups [95% vs 94%] as were overall and serious adverse event rates including renal dysfunction, liver enzyme elevations and osteopenia; marked fasting LDL cholesterol elevations were more frequent with the single tablet compared to control regimen [7% vs 2%]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Emtricitabine: a novel nucleoside reverse transcriptase inhibitor.[Drugs Today (Barc). 2005]Review Emtricitabine: a novel nucleoside reverse transcriptase inhibitor.Molina JM, Cox SL. Drugs Today (Barc). 2005 Apr; 41(4):241-52.
- Review Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: a review.[World J Gastroenterol. 2014]Review Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: a review.Sun HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. World J Gastroenterol. 2014 Oct 28; 20(40):14598-614.
- A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B.[Arch Intern Med. 2006]A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B.Lim SG, Ng TM, Kung N, Krastev Z, Volfova M, Husa P, Lee SS, Chan S, Shiffman ML, Washington MK, et al. Arch Intern Med. 2006 Jan 9; 166(1):49-56.
- Review Treatment of chronic hepatitis B in HIV co-infected patients.[Med J Malaysia. 2005]Review Treatment of chronic hepatitis B in HIV co-infected patients.Guan R. Med J Malaysia. 2005 Jul; 60 Suppl B:52-6.
- Management of the Hepatitis B Virus/HIV-Coinfected Patient.[Top Antivir Med. 2015]Management of the Hepatitis B Virus/HIV-Coinfected Patient.Sherman KE. Top Antivir Med. 2015 Aug-Sep; 23(3):111-4.
- Emtricitabine - LiverToxEmtricitabine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...