NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Dantrolene is a muscle relaxant used for treatment of chronic spasticity that differs from other commonly used muscle relaxants in acting peripherally on muscle, rather than centrally on the spinal cord or brain. Dantrolene can cause acute liver injury which can be severe and even fatal.
Background
Dantrolene (dan' troe leen) is a lipid soluble diphenylhydantoin analogue that inhibits muscle contractions by decreasing the release of calcium from the sarcoplasmic reticulum in target tissue. Dantrolene is used for the treatment of chronic spasticity and treatment for and prophylaxis against malignant hyperthermia (based upon its ability to block calcium release, which is the initiating event in malignant hyperthermia). Dantrolene was approved for use in the United States in 1974 and is still commonly used for spasticity. Dantrolene is available as capsules of 25, 50 and 100 mg in several generic forms and under the commercial name Dantrium. In adults, the recommended initial dose for spasticity is 25 mg daily, with subsequent increases to a dose of 25 to 100 mg three times daily. Dantrolene is also available in parenteral formulations for therapy of acute episodes of malignant hyperthermia; the recommended initial dose being 1 mg/kg intravenously. For prophylaxis against hyperthermia, dantrolene is given orally in doses of 4 to 8 mg/kg daily. Common side effects include weakness, nausea, drowsiness, fatigue and dizziness.
Hepatotoxicity
Mild, asymptomatic serum aminotransferase elevations during dantrolene therapy are relatively uncommon (1%), but clinically over liver injury is estimated to occur in 1 to 2 per thousand treated persons (0.1% to 0.2%). The liver injury can be severe; cases of acute liver failure and even death have been described (Case 1). The latency to onset of clinically apparent liver injury ranges from one week to several months, but is usually within the first 6 months of starting therapy (Case 2). More serious cases are associated with a sudden onset with jaundice, nausea and fatigue, and rapid progression. Allergic manifestations such as fever, rash and eosinophilia are rare, as are autoimmune features. The pattern of enzyme elevations is predominantly hepatocellular. Liver histology demonstrates an acute-hepatitis like picture. Recovery is usually complete within 1 to 3 months. Women, the elderly, and patients taking higher doses appear to be more susceptible to developing dantrolene hepatotoxicity.
Likelihood score: A (Well established cause of clinically apparent liver injury).
Mechanism of Injury
Dantrolene is structurally similar to phenytoin, which is a well known cause of idiosyncratic acute liver injury; thus, two agents may share a similar mechanism of hepatic injury. However, dantrolene rarely causes immunoallergic hepatitis (which is typical of phenytoin) and cases of more severe injury are associated with taking higher doses of dantrolene, suggesting the presence of a toxic metabolite.
Outcome and Management
Cases of acute hepatitis with jaundice due to dantrolene can be severe and even fatal; however, most cases are followed by complete recovery within 1 to 3 months of stopping the medication. Cases of chronic hepatitis or bile duct vanishing syndrome have not been reported. Rechallenge results in recurrence and should be avoided. There is at least a theoretical concern of cross sensitivity with phenytoin, but other drugs for spasticity should be tolerated without recurrence of liver injury.
Drug Class: Muscle Relaxants
CASE REPORTS
Case 1. Fatal progressive liver disease after a 12 week course of dantrolene.
[Modified from: Cornette M, Gillard C, Borlee-Hermans G. (Fatal toxic hepatitis associated with administration of dantrolene.) Acta Neurol Belg 1980; 80: 336-47. French. PubMed Citation]
A 50 year old woman with diabetes and multiple sclerosis was given dantrolene for spasticity and developed jaundice 12 weeks later. She began complaining of nausea, poor appetite, and weight loss after 7 weeks of therapy, but dantrolene was continued and she had lost 13 kilograms of weight and developed jaundice before the drug was stopped. She had no history of liver disease or exposure to hepatitis and did not drink alcohol. On admission, she was mildly jaundiced and laboratory tests showed mild elevations in serum ALT and alkaline phosphatase (Table). There was no fever or rash. Tests for viral hepatitis and autoantibodies were negative. Cholangiography was negative as was a CT scan of the liver. Nevertheless, she underwent exploratory laparotomy. Intraoperative liver biopsy showed intrahepatic cholestasis and mixed cellular injury suggestive of drug induced liver disease. She deteriorated postoperatively with progressive jaundice, ascites, and hepatic encephalopathy, dying of multiorgan failure 2 weeks after surgery.
Key Points
| Medication: | Dantrolene (400 mg daily) |
|---|---|
| Pattern: | Mixed (R=~2.1) |
| Severity: | 5+ (death) |
| Latency: | 95 days |
| Recovery: | None |
| Other medications: | Not mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| -1 month | 20 | 75 | 0.5 | ||
| 0 | Start Dantrolene | ||||
| 1 month | 33 | 147 | |||
| 2 months | Onset of symptoms | ||||
| 3 months | 0 | 57 | 175 | 2.0 | Jaundice, dantrolene stopped |
| 17 days | 73 | 135 | 9.0 | Exploratory laparotomy | |
| 24 days | 214 | 123 | 25.0 | Ascites, encephalopathy | |
| 31 days | 159 | 414 | 35.0 | ||
| 4 months | 32 days | Death | |||
| Normal Values | <42 | <115 | <1.2 | ||
Comment
This patient was continued on dantrolene despite symptoms of liver disease for several weeks and, perhaps as a consequence, had a progressive downhill course with worsening jaundice and hepatic synthetic dysfunction. Also unfortunate, was the unnecessary laparotomy and general anesthesia which may have worsened the liver injury. Somewhat unusual in this case was the minimal elevations in serum ALT at presentation, but there may have been more marked elevations earlier in the symptomatic course before clinical evaluation.
Case 2. Acute hepatitis from dantrolene with recurrence on rechallenge.
[Modified from: Ogburn RM, Myers RL, Burdick GE. Hepatitis associated with dantrolene sodium. Ann Intern Med 1976; 84: 53-4. PubMed Citation]
A 26 year old man with Friedreich's ataxia with spasticity and dysarthria was started on dantrolene in a dose of 25 mg four times daily. After 10 days, his dysarthria worsened and dantrolene was stopped. Serum enzymes were reported to be normal. One year later, dantrolene was restarted and he again experienced worsening dysarthria and stopped the medication after 10 days. Over the course of the following month, he had the insidious onset of malaise, nausea, dark urine and light stools. When found to be jaundiced, he was admitted for evaluation. He denied a history of liver disease and exposure to viral hepatitis or other toxins. Total bilirubin was 4.6 mg/dL, AST 740 U/L, but alkaline phosphatase was normal (Table). Tests for viral hepatitis and autoantibodies were negative. He had no rash, fever or eosinophilia. He improved rapidly and was discharged. Two months after recovering, he was restarted on dantrolene by another physician. His dysarthria worsened and, for the third time, he stopped the medication after 10 days. The day after stopping therapy, serum AST was 115 U/L and bilirubin was slightly elevated. During the next few weeks, he developed clinically apparent acute liver injury and was admitted. Laboratory tests again showed no evidence of viral hepatitis or autoimmune liver condition. A liver biopsy showed an acute hepatitis-like picture with prominence of eosinophils in portal areas. Over the next two weeks, the patient recovered and was discharged. Two weeks later he was clinically well and serum AST had fallen to 96 U/L.
Key Points
| Medication: | Dantrolene (100 mg daily) |
|---|---|
| Pattern: | Hepatocellular (ALT without Alk P elevations) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | Initially, ~33 days from starting, 23 days after stopping the drug. On rechallenge, ~11 days from starting, 1 day after stopping |
| Recovery: | Complete |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 10 days | 0 | Dantrolene stopped due to dysarthria | |||
| 33 days | 23 days | Malaise, nausea, vomiting, pale stools | |||
| 42 days | 32 days | 740 | 106 | 4.6 | Jaundice |
| 43 days | 34 days | 500 | Discharged | ||
| 68 days | 58 days | 52 | 1.6 | ||
| Time After Restarting | Time After Stopping Again | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Dantolene Restarted |
| 10 days | 0 | Dantrolene stopped because of worsening dysarthria | |||
| 11 days | 1 day | 115 | 2.1 | Jaundice | |
| 24 days | 2 weeks | 1150 | 4.5 | Acute hepatitis like symptoms | |
| 4 weeks | 3 weeks | Biopsy | |||
| 6 weeks | 5 weeks | 96 | |||
| Normal Values | <42 | <115 | <1.2 | ||
Comment
The recurrence of an acute hepatitis-like syndrome with rechallenge and similarity of the two episodes with a shortening of the latency on second exposure is strongly supportive of the role of dantrolene in causing the acute hepatitis-like liver injury. Somewhat unusual was the worsening of the liver injury for several weeks after dantrolene was stopped. Dantrolene, by inhibiting calcium release from the sarcoplasmic reticulum, can also cause generalized weakness which may account for the dysarthria that this patient experienced with each exposure to dantrolene. By limiting the duration of therapy, the dysarthria probably spared him a more severe hepatic injury.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Dantrolene – Generic, Dantrium®
DRUG CLASS
Autonomic Agents: Muscle Relaxants, Central
Product labeling at DailyMed, National Library of Medicine, NIH
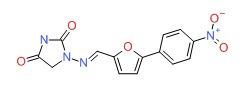
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Dantrolene | 7261-97-4 | C14-H10-N4-O5 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 30 January 2017
- Zimmerman HJ. Muscle spasmolytics. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 544-45.(Expert review of hepatotoxicity published in 1999 mentions that dantrolene causes overt liver injury in 0.4% of recipients, which is usually hepatocellular and sometimes fatal).
- Chyatte SB, Birdsong JH. The use of dantrolene sodium in disorders of the central nervous system. South Med J 1971; 64: 830-4. [PubMed: 4933014](Among 30 patients treated with dantrolene long term, 3 developed transient AST elevations but none were considered clinically significant).
- Herman R, Mayer N, Mecomber SA. Clinical pharmaco-physiology of dantrolene sodium. Am J Phys Med 1972; 51: 296-311. [PubMed: 4564128](Study of effects of dantrolene on neuromuscular activity in humans; no mention of adverse effects).
- Mayer N, Necomber SA, Herman R. Treatment of spasticity with dantrolene sodium. Am J Phys Med 1973; 52:18-29. [PubMed: 4685519](A study of efficacy and safety of dantrolene; one patient with alcoholic cirrhosis had mild ALT and AST elevations that resolved with stopping and recurred with restarting drug).
- Joynt RL. Dantrolene sodium: long-term effects in patients with muscle spasticity. Arch Phys Med Rehabil 1976; 57: 212-7. [PubMed: 776122](Among 77 patients treated with dantrolene for up to 2 years, 3 had AST elevations and one developed jaundice after 2 months and died one week later apparently of liver failure).
- Ogburn RM, Myers RL, Burdick GE. Letter: Hepatitis associated with dantrolene sodium. Ann Intern Med 1976; 84: 53-4. [PubMed: 1244796](26 year old man developed acute hepatitis like syndrome 43 days after starting dantrolene [bilirubin 4.6 mg/dL, ALT 740 U/L, Alk P 106 U/L], resolving in a few weeks; recurrence with latency of 11 days on reexposure [Case 2]).
- Schneider R, Mitchell D. Dantrolene hepatitis. JAMA 1976; 235:1590-1. [PubMed: 946274](38 year old woman with spasticity developed jaundice 16 weeks after starting escalating doses of dantrolene [bilirubin 9.0 rising to 32.8 mg/dL, AST 258 U/L, Alk P 215 U/L], resolving within 3 months of stopping).
- Assatourians P. Dantrolene hepatitis. JAMA 1976; 236: 1351. [PubMed: 989080](Letter in response to Schneider [1976] arguing that a liver biopsy rather than ERCP was the diagnostic procedure of choice: "Any student of hepatology knows...").
- Pinder RM, Brogden RN, Speight TM, Avery GS. Dantrolene sodium: a review of its pharmacological properties and therapeutic efficacy in spasticity. Drugs 1977; 13: 3-23. [PubMed: 318989](Review of efficacy and safety of dantrolene; minor abnormalities in AST occur in 10% of patients and hepatitis in 0.35-0.5%).
- Utili R, Boitnott JK, Zimmerman HJ. Dantrolene-associated hepatic injury. Incidence and character. Gastroenterology 1977; 72 (4 Pt 1): 610-6. [PubMed: 838213](A study of dantrolene safety based upon 2191 patients treated for at least 60 days; 1.8% developed evidence of hepatic injury, 0.6% jaundice and 0.3% died: about 1% had to stop due to hepatotoxicity and 2 of 5 who were restarted had a recurrence; recovery time ranged from 1 to 12 months and women were more likely to have liver injury than men; analysis of ~30,000 patients taking long term dantrolene revealed 31 physician reported cases of hepatoxicity [0.1%], 16 [52%] being jaundiced and 11 [35%] fatal).
- Wilkinson SP, Portmann B, Williams R. Hepatitis from dantrolene sodium. Gut 1979; 20: 33-6. [PMC free article: PMC1418951] [PubMed: 761834](Description of 1 man and 3 women [ages 45 to 80 years] who developed hepatotoxicity 1 to 3 months after starting dantrolene [bilirubin normal to 19 mg/dL, AST 137 to 800 U/L, Alk P 117 to 345 U/L], liver biopsy revealed acute hepatitis with focal or bridging necrosis, recovery in 4 to 12 weeks).
- Cornette M, Gillard C, Borlee-Hermans G. [Fatal toxic hepatitis associated with administration of dantrolene (author's transl)] Acta Neurol Belg 1980; 80: 336-47. French. [PubMed: 7468146](50 year old woman developed nausea, weight loss, and jaundice 3 months after starting dantrolene [bilirubin 2.0 rising to 35 mg/dL, ALT 57-214 U/L and Alk P 175-414 U/L], undergoing cholecystectomy and dying of sepsis and shock several days later [Case 1]).
- Arnold TH Jr, Epps JM 3rd, Cook HR, Hamrick ME. Dantrolene sodium: urinary metabolites and hepatotoxicity. Res Commun Chem Pathol Pharmacol 1983; 39: 381-98. [PubMed: 6856946](Animal study showing dose related hepatotoxicity of dantrolene in mice).
- Durham JA, Gandolfi AJ, Bentley JB. Hepatotoxicological evaluation of dantrolene sodium. Drug Chem Toxicol 1984; 7: 23-40. [PubMed: 6723545](Animal study showing no hepatotoxicity of dantrolene in mice).
- Sorensen EM, Acosta D. Comparison of dantrolene sodium with erythromycin estolate using primary cultures of rat hepatocytes. Drug Chem Toxicol 1985; 8: 219-37. [PubMed: 4075997](Erythromycin, but not dantrolene, caused injury to primary rat hepatocytes).
- Chan CH. Dantrolene sodium and hepatic injury. Neurology. 1990; 40: 1427-32. [PubMed: 2392230](Review of 122 cases of dantrolene hepatotoxicity reported to manufacturer; 59 with symptoms, 36 jaundice and 27 fatal; most arising within first 6 months, but fatal cases often late; mean ALT 784 U/L, Alk P 2-3 times ULN).
- Tolman KG. Hepatotoxicity of antirheumatic drugs. J Rheumatol Suppl 1990; 22: 6-11. [PubMed: 2192059](Review of hepatotoxicity of antirheumatic drugs including dantrolene).
- Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage 2004; 28: 140-75. [PubMed: 15276195](Thorough review of the pharmacology, efficacy and side effects of the muscle relaxants).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to dantrolene or other muscle relaxants).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, one was attributed to chlorzoxazone, but none to dantrolene or other muscle relaxants).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to dantrolene).
- Kim JY, Chun S, Bang MS, Shin HI, Lee SU. Safety of low-dose oral dantrolene sodium on hepatic function. Arch Phys Med Rehabil 2011; 92: 1359-63. [PubMed: 21878205](Retrospective analysis of liver test results from 243 patients treated with dantrolene for 4 weeks or more; one patient, an HBsAg carrier, developed elevations in liver tests without symptoms 82 days after starting dantrolene [bilirubin rising from 0.5 to 2.5 mg/dL, AST 54 to 85 U/L, Alk P 167 to 245 U/L], which resolved on stopping).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013 ; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to dantrolene or other muscle relaxants).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to dantrolene or other muscle relaxants).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 [0.7%] were attributed to muscle relaxants including 2 to dantrolene).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Dantrolene sodium: a review of its pharmacological properties and therapeutic efficacy in spasticity.[Drugs. 1977]Review Dantrolene sodium: a review of its pharmacological properties and therapeutic efficacy in spasticity.Pinder RM, Brogden RN, Speight TM, Avery GS. Drugs. 1977 Jan; 13(1):3-23.
- Oral dantrolene and severe respiratory failure in a patient with chronic spinal cord injury.[Anaesthesia. 2010]Oral dantrolene and severe respiratory failure in a patient with chronic spinal cord injury.Javed M, Bogdanov A. Anaesthesia. 2010 Aug; 65(8):855-6. Epub 2010 Jun 17.
- Review Spasticity and drug therapy.[Pharm Weekbl Sci. 1987]Review Spasticity and drug therapy.Wuis EW. Pharm Weekbl Sci. 1987 Oct 16; 9(5):249-60.
- The effect of dantrolene sodium in relation to blood levels in spastic patients after prolonged administration.[J Neurol Neurosurg Psychiatry....]The effect of dantrolene sodium in relation to blood levels in spastic patients after prolonged administration.Meyler WJ, Bakker H, Kok JJ, Agoston S, Wesseling H. J Neurol Neurosurg Psychiatry. 1981 Apr; 44(4):334-9.
- Mechanism of action of dantrolene sodium, a peripherally acting muscle relaxant.[Electroencephalogr Clin Neurop...]Mechanism of action of dantrolene sodium, a peripherally acting muscle relaxant.Endo M, Yagi S. Electroencephalogr Clin Neurophysiol Suppl. 1982; 36:216-20.
- Dantrolene - LiverToxDantrolene - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...