NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Cobimetinib is a tyrosine kinase receptor inhibitor that is used in combination with vemurafenib as therapy for selected forms of advanced malignant melanoma. The combination of cobimetinib and vemurafenib is commonly associated with serum enzyme elevations during therapy and to rare instances of clinically apparent acute liver injury.
Background
Cobimetinib (koe" bi me' ti nib) is a small molecule tyrosine kinase receptor inhibitor with potent activity against the mitogen-activated extracellular regulated kinase (MEK), which is an important component of the kinase cascade in the mitogen activated protein kinase (MAPK) pathway (RAS-RAF-MEK-ERK). Components of the MAPK pathways are frequently mutated in patients with malignant melanoma, particularly the RAF isoform BRAF. BRAF-mutations cause a constitutive activation of the MAPK pathways, resulting in unregulated cell growth and proliferation. Inhibition of MEK has been found to be synergistic when combined with specific BRAF inhibitors as therapy of BRAF-mutated cancers. Clinical trials have shown that the addition of cobimetinib with vemurafenib (a specific BRAF-kinase inhibitor) results in improvements in survival in patients with melanoma who harbor the V600 BRAF mutation. Cobimetinib received accelerated approval for use as combination therapy with vemurafenib in the United States in 2015. Current indications are as combination therapy of metastatic or unresectable melanoma with BRAF V600E or V600K mutations. Cobimetinib is available in tablets of 20 mg under the brand name Cotellic. The recommended dose is 60 mg once daily for the first 21 days of each 28-day cycle, continued until disease progression or intolerable toxicity occurs. Side effects are common and include diarrhea, rash, photosensitivity, nausea, stomatitis, fever, alopecia and thrombocytopenia. Uncommon, but potentially severe side effects include severe diarrhea leading to dehydration and renal failure, rhabdomyolysis, cardiac toxicity, retinal detachment and embryo-fetal toxicity.
Hepatotoxicity
Elevations in serum aminotransferase and alkaline phosphatase levels are common during vemurafenib therapy and are even more common when it is combined with cobimetinib, abnormal liver tests occurring in 26% to 70% of treated patients and ALT values rising above 5 times the upper limit of the normal range (ULN) in 6% to 12%. Instances of clinically apparent liver injury with jaundice have also been reported during the clinical trials of cobimetinib and vemurafenib therapy, but the clinical features, course and outcomes of these episodes have not been described in detail. At least one instance of hepatocellular injury with jaundice was included in the initial safety review of cobimetinib. The MAPK pathway inhibitors as a class are often associated with transient serum enzyme elevations and more rarely with instances of clinically apparent liver injury, but the clinical features have not been described and the association with cobimetinib not clearly defined. The rate of clinically significant liver injury and hepatic failure associated with protein kinase inhibitors is increased in patients with preexisting cirrhosis or hepatic impairment due to liver tumor burden.
Likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
The cause of liver injury that occurs during therapy with MAPK pathway inhibitors is not well understood, but may relate to the direct effects of inhibition of these enzymes on hepatocytes. Cobimetinibb is metabolized in the liver via the cytochrome P450 system, predominantly CYP 3A and is susceptible to drug-drug interactions with strong inhibitors or inducers of this microsomal enzyme.
Outcome and Management
Liver injury due to cobimetinib varies in severity from minor, transient serum enzyme elevations to acute symptomatic hepatitis. The product label for cobimetinib recommends monitoring of routine liver tests during treatment. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to temporary discontinuation, which should be permanent if laboratory values do not improve significantly or resolve within a few weeks. Restarting therapy is usually, but not always followed by recurrence of the serum enzyme elevations. There does not appear to be cross reactivity with other tyrosine kinase receptor inhibitors and, in some situations, switching to another protein kinase inhibitor may be appropriate.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
Related Drugs: Trametinib, Vemurafenib
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cobimetinib – Cotellic®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
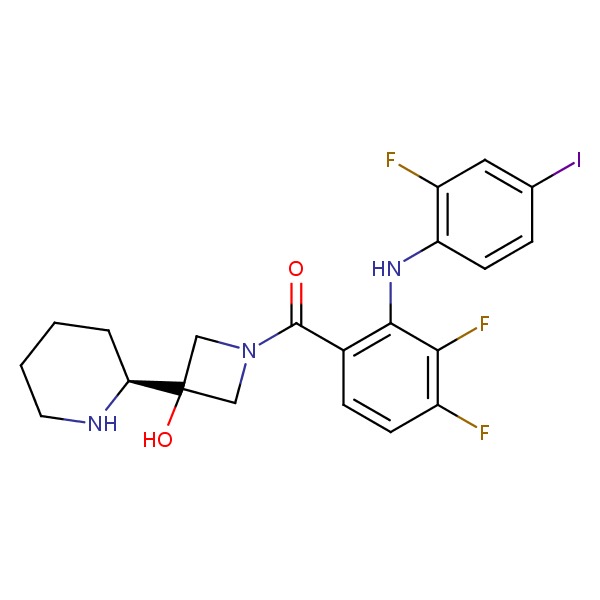
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Cobimetinib | 934660-93-2 | C21-H21-F3-I-N3-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 01 June 2017
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase receptor inhibitors).
- DeLeve LD. Kinase inhibitors. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556-7.(Review of hepatotoxicity of cancer chemotherapeutic agents; discusses gefitinib, erlotinib and crizotinib but not cobimetinib).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-54.(Textbook of pharmacology and therapeutics).
- Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, Mandalà M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371: 1867-76. [PubMed: 25265494](Among 495 patients with untreated, unresectable or metastatic BRAF V600 mutation-positive melanoma treated with vemurafenib with vs without cobimetinib, overall and progression free survival was greater with the combination as were adverse events with ALT elevations in 24% vs 19% that were above 5 times ULN in 12% vs 7%).
- Ribas A, Gonzalez R, Pavlick A, Hamid O, Gajewski TF, Daud A, Flaherty L, et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAF (V600)-mutated melanoma: a phase 1b study. Lancet Onco. 2014; 15: 954-65. [PubMed: 25037139](Among 129 patients with advanced, BRAF V600 mutated melanoma treated with 10 different combination regimens of vemurafenib and cobimetinib, dose limiting toxicities were fatigue, QTc interval prolongation, stomatitis and myalgia and arthralgia while liver test abnormalities arose in 50% of patients, with ALT elevations in 21%, that were above 5 times ULN in 5%).
- Patel U, Cornelius L, Anadkat MJ. MEK inhibitor-induced dusky erythema: characteristic drug hypersensitivity manifestation in 3 patients. JAMA Dermatol 2015; 151: 78-81. [PubMed: 25426865](Three patients with advanced malignancies developed skin rashes and evidence of drug hypersensitivity within 1-7 weeks of starting therapy with 3 different MEK-inhibitors [selumetinib, cobimetinib and trametinib], resolving with stopping and, in two instances, after corticosteroid therapy, not recurring upon restarting the same agent).
- Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, Di Giacomo AM, Mandalà M, et al. Cobimetinibb combined with vemurafenib in advanced BRAF (V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016; 17: 1248-60. [PubMed: 27480103](Follow up of study of Larkin et al [2014] in 495 patients with advanced BRAF-V600 mutant melanoma, found median overall survival was greater with the combination of cobimetinib and vemurafenib vs vemurafenib alone [22.3 vs 17.4 months] as were serious adverse events [37% vs 28%], any ALT elevation [26% vs 18%] and ALT elevations above 5 times ULN [11% vs 6%]).
- Cobimetinibb (Cotellic) for metastatic melanoma. Med Lett Drugs Ther 2016; 58 (1491): 43-4. [PubMed: 27027689](Concise review of the mechanism of action, pharmacology, clinical efficacy, toxicity and costs of cobimetinib shortly after its approval in the US; mentions that serum enzyme elevations are common during cobimetinib therapy, but does not mention clinically apparent liver injury or hepatic failure).
- Rosen LS, LoRusso P, Ma WW, Goldman JW, Weise A, Colevas AD, Adjei A, et al. A first-in-human phase I study to evaluate the MEK1/2 inhibitor, cobimetinib, administered daily in patients with advanced solid tumors. Invest New Drugs 2016; 34: 604-13. [PMC free article: PMC6863157] [PubMed: 27424159](Among 97 patients with various forms of advanced malignancies treated with different doses and regimens of cobimetinib in a phase 1 study, dose-limiting toxicities included hepatic encephalopathy, diarrhea, rash and blurred vision while common adverse events were diarrhea, rash, fatigue, edema, nausea; clinical responses occurred only in patients with melanoma, most of whom had BRAF V600E mutations).
- Signorelli J, Shah Gandhi A. Cobimetinibb. Ann Pharmacother 2017; 51: 146-53. [PubMed: 27701080](Review of the pharmacology, efficacy and safety of cobimetinib in combination with vemurafenib; mentions that the most frequent adverse events are rash [87%], diarrhea [83%], fatigue [70%], photosensitivity [67%], and liver enzyme elevations [67%]; no mention of clinically apparent liver injury or liver related mortality).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study.[Lancet Oncol. 2014]Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study.Ribas A, Gonzalez R, Pavlick A, Hamid O, Gajewski TF, Daud A, Flaherty L, Logan T, Chmielowski B, Lewis K, et al. Lancet Oncol. 2014 Aug; 15(9):954-65. Epub 2014 Jul 15.
- Review Atezolizumab, cobimetinib, and vemurafenib as first-line treatment for unresectable metastatic BRAF V600 mutated melanoma.[Expert Rev Anticancer Ther. 2022]Review Atezolizumab, cobimetinib, and vemurafenib as first-line treatment for unresectable metastatic BRAF V600 mutated melanoma.Schmitt AM, Dumas L, Larkin J. Expert Rev Anticancer Ther. 2022 Jan; 22(1):17-25. Epub 2022 Jan 2.
- Review Cobimetinib.[Ann Pharmacother. 2017]Review Cobimetinib.Signorelli J, Shah Gandhi A. Ann Pharmacother. 2017 Feb; 51(2):146-153. Epub 2016 Oct 4.
- Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial.[Lancet Oncol. 2016]Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial.Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, Di Giacomo AM, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, et al. Lancet Oncol. 2016 Sep; 17(9):1248-60. Epub 2016 Jul 30.
- Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study.[Ann Oncol. 2017]Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study.Dréno B, Ribas A, Larkin J, Ascierto PA, Hauschild A, Thomas L, Grob JJ, Koralek DO, Rooney I, Hsu JJ, et al. Ann Oncol. 2017 May 1; 28(5):1137-1144.
- Cobimetinib - LiverToxCobimetinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...