NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Cholic acid is a naturally occurring bile acid that is used to treat patients with genetic deficiencies in the synthesis of bile acids. When given in high doses, cholic acid replacement therapy has been linked to minor elevations in serum aminotransferase levels, but it has not been linked to instances of clinically apparent acute liver injury with jaundice.
Background
Cholic (koe' lik) acid is a naturally occurring, primary bile acid that represents a major component of the total bile acid pool in humans. Cholic acid is synthesized from cholesterol in the liver and is conjugated to either glycine (glycocholic acid) or taurine (taurocholic acid) before secretion in the bile. Bile acids act in bile to solubilize cholesterol (which is totally insoluble in water) with phospholipids in mixed micelles. Once secreted into the intestine, bile acids help to emulsify fats and aid in their digestion. Cholic acid is also a potent signaling molecule and acts as a weak FXR agonist resulting in a decrease in bile acid synthesis and parallel increase in cholesterol. For these reasons, cholic acid is not effective in gallstone dissolution. Cholic acid is used therapeutically to treat patients with bile acid synthetic defects due to single enzyme deficiencies. Defects in several of the enzymes involved in bile acid synthesis can result in the production of abnormal and insufficient levels of bile acids with resultant fat malabsorption and progressive cholestatic liver disease. Cholic acid administration reverses most of these abnormalities. Cholic acid was given orphan drug approval for use in the United States for treatment of children and adults with bile acid synthetic defects and Zellweger’s syndrome (a peroxisomal disorder characterized by progressive liver disease and malabsorption) in 2015. A glycine conjugate of cholic acid (glycocholic acid) is currently being evaluated for treatment of bile acid conjugation defects. Cholic acid is available as capsules of 50 and 250 mg under the brand name Cholbam. The typical dose is 10 to 15 mg/kg once daily. Side effects are largely dose related and include diarrhea, indigestion, nausea, abdominal discomfort, fatigue, and skin rash.
Hepatotoxicity
In small, open label trials, cholic acid was found to improve fat soluble vitamin absorption and to ameliorate many of the clinical features of the bile acid synthetic defects including improvement in serum aminotransferase levels, decrease in bilirubin and jaundice and improvement in general health and growth. In some instances, higher doses of cholic acid were associated with elevations in serum aminotransferase levels. These abnormalities, however, were mild, transient and rapidly reversed with lowering the daily dose. There have been no reports of clinically apparent liver injury with jaundice attributed to cholic acid therapy given in standard doses.
Likelihood score: D[HD] (possible cause of liver injury but only when given in high doses).
Mechanism of Liver Injury
The mechanism by which high doses of cholic acid might cause liver injury is unclear. An increase in cholic acid levels in hepatocytes may be mildly hepatotoxic.
Other bile acids used in digestive diseases include chenodeoxycholic acid (chenodiol), obeticholic acid and ursodeoxycholic acid (ursodiol).
Drug Class: Gastrointestinal Agents, Bile Acids
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cholic Acid – Cholbam®
DRUG CLASS
Gastrointestinal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Cholic Acid | 81-25-4 | C24-H40-O5 |
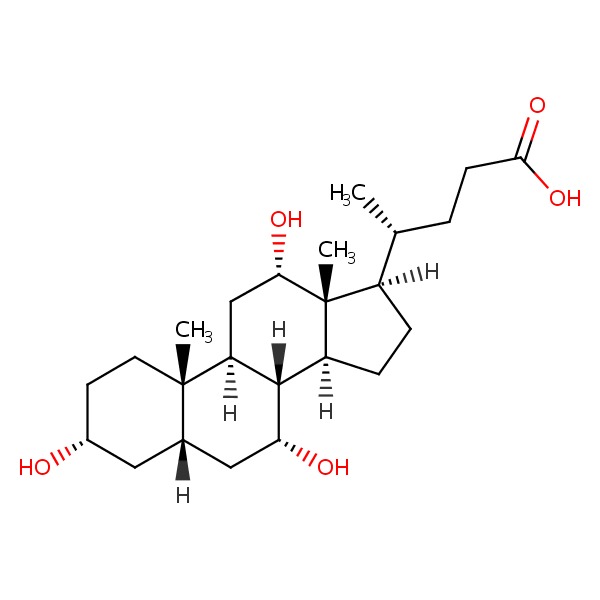 |
ANNOTATED BIBLIOGRAPHY
References updated: 07 November 2016
- Zimmerman HJ. Bile acid derivatives. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 721.(Expert review of hepatotoxicity published in 1999 before the availability of cholic acid).
- Sharkey KA, Wallace JL. Treatment of disorders of bowel motility and water flux: anti-emetics; agents used in biliary and pancreatic disease. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1323-50.(Textbook of pharmacology and therapeutics).
- Daugherty CC, Setchell KD, Heubi JE, Balistreri WF. Resolution of liver biopsy alterations in three siblings with bile acid treatment of an inborn error of bile acid metabolism (delta 4-3-oxosteroid 5 beta-reductase deficiency). Hepatology 1993; 18: 1096-101. [PubMed: 8225213](Among 3 siblings with bile acid synthetic defects and severe liver disease, treatment with cholic acid and ursodiol was followed by resolution of jaundice and improvements in ALT, Alk P and liver histology).
- Bove KE, Heubi JE, Balistreri WF, Setchell KD. Bile acid synthetic defects and liver disease: a comprehensive review. Pediatr Dev Pathol 2004; 7: 315-34. [PubMed: 15383928](Extensive review of the inherited forms of defective bile acid synthesis which are often accompanied by progressive cholestatic liver injury, some of which respond to treatment with oral bile acids, including 3β-OH steroid dehydrogenase, 5β-reductase, and mitochondrial C-27 hydroxylase deficiencies as well as several peroxisomal defects [Zellweger syndrome]).
- Woollett LA, Buckley DD, Yao L, Jones PJ, Granholm NA, Tolley EA, Tso P, Heubi JE. Cholic acid supplementation enhances cholesterol absorption in humans. Gastroenterology 2004; 126: 724-31. [PubMed: 14988826](In a crossover design study in healthy volunteers, administration of cholic acid [15 mg/kg daily] resulted in an increase in cholesterol absorption).
- Chen J, Raymond K. Nuclear receptors, bile-acid detoxification, and cholestasis. Lancet 2006; 367(9509): 454-6. [PubMed: 16473109](Commentary on the nuclear receptor FXR, which acts as a bile acid receptor and mediates reduction in bile acid synthesis and increase in uptake of bile acids from the circulation as well as secretion of bile acids from hepatocytes).
- Wang Y, Jones PJ, Woollett LA, Buckley DD, Yao L, Granholm NA, Tolley EA, Heubi JE. Effects of chenodeoxycholic acid and deoxycholic acid on cholesterol absorption and metabolism in humans. Transl Res 2006; 148: 37-45. [PubMed: 16887497](In two cross over studies in adults, oral chenodiol had no effect of plasma lipid concentrations while it increased biliary chenodiol, but also increased biliary cholesterol concentrations).
- Heubi JE, Setchell KD, Bove KE. Inborn errors of bile acid metabolism. Semin Liver Dis 2007; 27: 282-94. [PubMed: 17682975](Review of the rare inherited conditions marked by abnormal bile acid synthesis or metabolism, the diagnosis of which generally requires mass spectrometry techniques, and many of which are treatable using oral bile acids such as cholic acid, chenodiol, ursodiol and glycocholic acid).
- Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 2009; 50: 1955-66. [PMC free article: PMC2739756] [PubMed: 19346330](Review of the pathways of cholic and chenodeoxycholic acid synthesis and their regulation via FXR, FGF19, FGFR4 and CYP7A1).
- Gonzales E, Gerhardt MF, Fabre M, Setchell KD, Davit-Spraul A, Vincent I, Heubi JE, et al. Oral cholic acid for hereditary defects of primary bile acid synthesis: a safe and effective long-term therapy. Gastroenterology 2009; 137: 1310-1320. [PubMed: 19622360](Among 15 patients with bile acid synthesis defects treated with oral bile acids [ursodiol and cholic acid initially, and eventually cholic acid alone for all except one patient: 3-9 mg/kg daily] for 5-15 years, all had lasting clinical improvements shown by repeat liver biopsies in 14; overdose of cholic acid was marked by diarrhea, pruritus and increases in GGT and ALT that responded to dose modification).
- Heubi JE, Setchell KD, Jha P, Buckley D, Zhang W, Rosenthal P, Potter C, et al. Treatment of bile acid amidation defects with glycocholic acid. Hepatology 2015; 61: 268-74. [PMC free article: PMC4280294] [PubMed: 25163551](Treatment of 5 patients with bile acid amidation defects with glycine conjugated cholic acid [glycocholic acid; 15 mg/kg daily] resulted in improved absorption of fat soluble vitamins and growth improvement in all 3 prepubertal children with delayed growth).
- In brief: Cholic acid (Cholbam) for bile acid synthesis disorders. Med Lett Drugs Ther 2016; 58 (1493): 56. [PubMed: 27101212](Short review of the efficacy, safety and costs of cholic acid, shortly after its approval as an orphan drug in the therapy of bile acid synthesis disorders, mentions that its most common adverse event is diarrhea).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Bile Acids.[LiverTox: Clinical and Researc...]Review Bile Acids.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Oral Cholic Acid Is Efficacious and Well Tolerated in Patients With Bile Acid Synthesis and Zellweger Spectrum Disorders.[J Pediatr Gastroenterol Nutr. ...]Oral Cholic Acid Is Efficacious and Well Tolerated in Patients With Bile Acid Synthesis and Zellweger Spectrum Disorders.Heubi JE, Bove KE, Setchell KDR. J Pediatr Gastroenterol Nutr. 2017 Sep; 65(3):321-326.
- Role of farnesoid X receptor in the enhancement of canalicular bile acid output and excretion of unconjugated bile acids: a mechanism for protection against cholic acid-induced liver toxicity.[J Pharmacol Exp Ther. 2005]Role of farnesoid X receptor in the enhancement of canalicular bile acid output and excretion of unconjugated bile acids: a mechanism for protection against cholic acid-induced liver toxicity.Miyata M, Tozawa A, Otsuka H, Nakamura T, Nagata K, Gonzalez FJ, Yamazoe Y. J Pharmacol Exp Ther. 2005 Feb; 312(2):759-66. Epub 2004 Oct 1.
- Open-label Phase 3 Continuation Study of Cholic Acid in Patients With Inborn Errors of Bile Acid Synthesis.[J Pediatr Gastroenterol Nutr. ...]Open-label Phase 3 Continuation Study of Cholic Acid in Patients With Inborn Errors of Bile Acid Synthesis.Heubi JE, Setchell KDR. J Pediatr Gastroenterol Nutr. 2020 Apr; 70(4):423-429.
- Review Ursodiol (Ursodeoxycholic Acid).[LiverTox: Clinical and Researc...]Review Ursodiol (Ursodeoxycholic Acid).. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Cholic Acid - LiverToxCholic Acid - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...