NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Chlordiazepoxide is an orally available benzodiazepine used for therapy of anxiety disorders and alcohol withdrawal syndromes. As with other benzodiazepines, chlordiazepoxide is not associated with serum aminotransferase or alkaline phosphatase elevations during therapy, and clinically apparent liver injury from chlordiazepoxide has been reported but is rare.
Background
Chlordiazepoxide (klor" dye az" e pox' ide) is the prototype of benzodiazepines used in the therapy of anxiety and acute alcohol withdrawal. The antianxiety (anxiolytic) activity of the benzodiazepines is mediated by their ability to enhance gamma-aminobutyric acid (GABA) mediated inhibition of synaptic transmission through binding to the GABA A receptor. Chlordiazepoxide was approved in the United States in 1960 and for many years was one of the most prescribed medications. Currently, it no longer commonly used, having been replaced by benzodiazepines with more favorable pharmacokinetics, half-life and tolerance. Indications include anxiety disorders and alcohol withdrawal syndrome. Chlordiazepoxide is available in multiple generic forms and formerly under the brand name of Librium in capsules of 5, 10 and 25 mg. The recommended initial dose for adults is 5 to 10 mg three to four times per day, but higher doses are used for severe anxiety disorders. Chlordiazepoxide is also available for parenteral administration (100 mg/ampule) for use in acute anxiety, preoperative sedation and acute alcohol withdrawal syndromes. In addition, combinations of chlordiazepoxide with clidinium bromide or amitriptyline have been marketed under generic as well as brand names (Librax and Limbitrol). The most common side effects of chlordiazepoxide are dose related and include drowsiness, lethargy, ataxia, dysarthria and dizziness. Tolerance develops to these side effects, but tolerance may also develop to the anxiolytic effects. Chlordiazepoxide like all oral benzodiazepines has a boxed warning in its product label stressing (1) the risks of severe sedation and potentially fatal respiratory depression when combined with opiates, (2) with prolonged use, the risks of abuse, misuse, and addiction which can lead to overdose and death, and (3) with continued use, the risks of dependence and severe, potentially life-threatening withdrawal symptoms if discontinued suddenly. Benzodiazepines are all categorized as Schedule IV controlled substances, having potential for abuse, addiction, and dependence.
Hepatotoxicity
Chlordiazepoxide, as with other benzodiazepines, is rarely associated with serum ALT elevations, and clinically apparent liver injury from its use is rare. There have been at least ten case reports of acute liver injury from chlordiazepoxide, published largely before 1980. The latency to onset of acute liver injury was 1 to 4 months, and the pattern of liver enzyme elevations varied from hepatocellular to cholestatic and mixed. The injury was usually mild-to-moderate in severity and self-limited. Fever and rash were uncommon, as was autoantibody formation.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
Chlordiazepoxide is metabolized extensively in the liver and has a prolonged half-life. The liver injury from benzodiazepines is probably due to a rarely produced metabolic intermediate metabolite.
Outcome and Management
The case reports of hepatic injury due to benzodiazepines were marked by prompt and complete recovery upon stopping the medication, without evidence of residual or chronic injury. No cases of acute liver failure or chronic liver injury due to chlordiazepoxide have been described. There is little information about cross reactivity with other benzodiazepines, but some degree of cross sensitivity should be assumed.
Drug Class: Benzodiazepines, Antianxiety Agents
CASE REPORT
Case 1. Mild acute liver injury due to chlordiazepoxide.(1)
A 26 year old woman developed nausea, anorexia and fatigue 2 days after starting chlordiazepoxide (30 mg per day). She was given an injection of penicillin followed by several days of oral penicillin. Ten days later she developed jaundice and pruritus and chlordiazepoxide was stopped. She had no previous history of liver disease or known exposures to viral hepatitis. She had delivered a healthy, full-term baby shortly before starting the chlordiazepoxide. She had no known drug allergies. Physical examination showed jaundice and a tender liver, without fever or skin rash. Laboratory tests showed elevated serum bilirubin (5.0 mg/dL) and elevations in both serum alkaline phosphatase and aminotransferase levels (Table). She had severe pruritus that persisted for several weeks, even while her laboratory test results improved. A liver biopsy showed intra-hepatic cholestasis. Two months later her symptoms had resolved and all blood tests had returned to normal.
Key Points
| Medication: | Chlordiazepoxide |
|---|---|
| Pattern: | Cholestatic (R=0.3) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 2 days to symptoms, 11 days to jaundice |
| Recovery: | 2 months |
| Other medications: | Penicillin G |
Laboratory Values
| Time After Starting | Time After* Stopping | AST* (U/L) | Alk P* (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| 2 weeks | 0 | 51 | 69 | 5.0 | Admission |
| 3 weeks | 7 days | 45 | 50 | 4.4 | Liver biopsy |
| 4 weeks | 14 days | 55 | 47 | 4.3 | |
| 4.5 weeks | 18 days | 52 | 39 | 3.4 | |
| 5 weeks | 3 weeks | 54 | 35 | 2.8 | |
| 6 weeks | 4 weeks | 35 | 20 | 1.6 | |
| 7 weeks | 5 weeks | 21 | 1.2 | ||
| 8 weeks | 6 weeks | 15 | 1.1 | ||
| Normal Values | <35 | <13 | <1.2 | ||
- *
Some dates and values estimated from Figure 1
Comment
An acute cholestatic hepatitis arose within two weeks of starting chlordiazepoxide in a patient whose only other medical exposure was to a few days or penicillin. While both benzodiazepines and the penicillins can cause cholestatic hepatitis, penicillin induced liver injury is usually accompanied by other signs of hypersensitivity such as fever and rash. Cholestatic drug induced liver injury tends to be slower to resolve that hepatocellular injury.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Chlordiazepoxide – Generic, Librium® (Trade name discontinued)
DRUG CLASS
Benzodiazepines
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Chlordiazepoxide | 58-25-3 | C16-H14-Cl-N3-O |
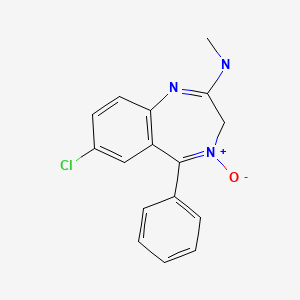
|
CITED REFERENCES
- 1.
- Lo KJ, Eastwood IR, Eidelman S. Cholestatic jaundice associated with chlordiazepoxide hydrochloride (Librium) therapy. Report of a case and review of the literature. Am J Dig Dis. 1967;12:845–9. [PubMed: 4952749]
ANNOTATED BIBLIOGRAPHY
References updated: 22 June 2023
- Zimmerman HJ. Benzodiazepines. Psychotropic and anticonvulsant agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 491-3.(Expert review of benzodiazepines and liver injury published in 1999; mentions rare instances of cholestatic hepatitis have been reported due to alprazolam, chlordiazepoxide, diazepam, flurazepam, and triazolam, and hepatocellular injury with clorazepate and clotiazepam, but no reports of hepatic injury with lorazepam, oxazepam, or temazepam).
- Larrey D, Ripault MP. Benzodiazepines. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 455.(Review of drug induced liver injury mentions that isolated instances of acute liver injury [usually cholestatic] have been reported with alprazolam, chlordiazepoxide, diazepam, flurazepam, and triazolam; a hepatitis-like pattern has been reported with clonazepam and clorazepate).
- Mihic SJ, Mayfield J, Harris RA. Hypnotics and sedatives. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 339-53.(Textbook of pharmacology and therapeutics).
- Cacioppo J, Merlis S. Chlordiazepoxide hydrochloride (Librium) and jaundice: report of a case. Am J Psychiatry. 1961;117:1040–1. [PubMed: 13689710](39 year old man with schizophrenia and seizures on phenytoin developed jaundice 5 days after starting chlordiazepoxide [50 mg/day] [icterus index 49.6, Alk P 3 times ULN], resolving within 3 months of stopping).
- Abbruzzese A, Swanson J. Jaundice after therapy with chlordiazepoxide hydrochloride. N Engl J Med. 1965;273:321–2. [PubMed: 21417069](51 year old man developed jaundice and pruritus 4-5 weeks after starting chlordiazepoxide [bilirubin ~4.0 mg/dL, AST 300 U/L, Alk P 2.5 times ULN], biopsy showed inflammation and cholestasis, recovery not mentioned).
- Pickering D. Hepatic necrosis after chlordiazepoxide therapy. N Engl J Med. 1966;274:1449.(64 year old woman developed jaundice 3 weeks after 12 day course of chlordiazepoxide [bilirubin ~32 mg/dL, ALT 225 U/L, Alk P 38 U/L], prolonged course, transient ascites, prednisone therapy).
- Lo KJ, Eastwood IR, Eidelman S. Cholestatic jaundice associated with chlordiazepoxide hydrochloride (Librium) therapy. Report of a case and review of the literature. Am J Dig Dis. 1967;12:845–9. [PubMed: 4952749](26 year old woman developed nausea after 2 and jaundice after 11 days of chlordiazepoxide therapy [bilirubin 5.0 mg/dL, AST 51 U/L, Alk K 69 KA ~5 times ULN], recovery over 8 weeks, marked pruritis: Case 1).
- Kratzsch KH, Buttner W, Reinhardt G. [Intrahepatic cholestasis following chlordiazepoxide--contribution to the differential diagnosis of drug jaundice]. Z Gesamte. Inn Med. 1972;27:408–11. German. [PubMed: 5055057](3 cases of cholestatic hepatitis during chlordiazepoxide use, after 4 weeks, 6 years and 2 days [bilirubin 6.0, 1.0 and 6.4 mg/dL, ALT 245, 18 and 25 U/L, Alk P 1.6 1.0 and 20 times ULN], with rapid recovery in 1-2 months, all 3 had biopsies showing intrahepatic cholestasis).
- Franks E, Jacobs WH. Cholestatic jaundice possibly due to benzodiazepine-type drugs. Mo Med. 1975;72:605–6. [PubMed: 1181510](40 year old woman on multiple drugs including chlorpromazine developed jaundice [bilirubin 2.0 rising to 9.7 mg/dL, ALT 280 U/L, Alk P 546 U/L, 16% eosinophils], seemed to worsen on benzodiazepines including chlordiazepoxide, diazepam and flurazepam, resolving rapidly when they were stopped, but attribution to benzodiazepines difficult).
- Døssing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol. 1982;17:205–11. [PubMed: 6982502](Among 572 cases of hepatotoxicity reported to a Danish registry between 1968 and 1978, 97 were due to psychotropic agents, but only two attributed to benzodiazepines).
- Davion T, Capron-Chivrac D, Andrejak M, Capron JP. Gastroenterol Clin Biol. 1985;9:117–26. [Hepatitis due to antiepileptic agents] [PubMed: 3920108](Review of hepatotoxicity of anticonvulsants; among benzodiazepines, cases of cholestatic hepatitis have been linked to chlordiazepoxide and diazepam, but liver injury from this class of drugs is exceptionally rare).
- Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf. 1996;15:378–93. [PubMed: 8968693](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine; “No child taking… benzodiazepines had raised liver enzyme levels,”).
- Lewis JH, Zimmerman HJ. Drug- and chemical-induced cholestasis. Clin Liver Dis. 1999;3:433–64. vii. [PubMed: 11291233](Review of drug induced cholestatic syndromes, listing many causes including chlordiazepoxide and flurazepam; “Benzodiazepines may cause cholestatic injury, although this is rare”).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Among 126 cases of drug induced liver injury seen in Spain between 1993-2000, 20 were attributed to benzodiazepines including 5 for clorazepate, 5 alprazolam, 6 lorazepam and 4 diazepam, but none attributed to chlordiazepoxide).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to a benzodiazepine).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118:281–90. [PubMed: 18341684](Review of hepatotoxicity of all anticonvulsants focusing upon phenytoin, valproate, carbamazepine; “Furthermore, hepatoxicity has not been convincingly shown to be associated with the use of benzodiazepines”).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were linked benzodiazepines).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to chlordiazepoxide or other benzodiazepines).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature on drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to a chlordiazepoxide or other benzodiazepine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, no cases were attributed to chlordiazepoxide or any other benzodiazepine).
- Drugs for anxiety disorders. Med Lett Drugs Ther. 2019;61:121–6. [PubMed: 31386647](Concise review of drugs for anxiety including SSRIs, SNRIs and benzodiazepines including mechanism of action, clinical efficacy, safety, and costs; does not mention ALT elevations or hepatotoxicity).
- Drugs for chronic insomnia. Med Lett Drugs Ther. 2023;65:1–6. [PubMed: 36630579](Concise review of drugs for chronic insomnia mentions that tolerance and dependence can occur with use of benzodiazepines and their use should be discouraged, and that benzodiazepines are CNS suppressants and can impair next day performance including driving and cause complex behavior disorders, retrograde amnesia, dependence, tolerance, abuse and rebound insomnia; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Oxazepam.[LiverTox: Clinical and Researc...]Review Oxazepam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Phenobarbital compared to benzodiazepines in alcohol withdrawal treatment: A register-based cohort study of subsequent benzodiazepine use, alcohol recidivism and mortality.[Drug Alcohol Depend. 2016]Phenobarbital compared to benzodiazepines in alcohol withdrawal treatment: A register-based cohort study of subsequent benzodiazepine use, alcohol recidivism and mortality.Askgaard G, Hallas J, Fink-Jensen A, Molander AC, Madsen KG, Pottegård A. Drug Alcohol Depend. 2016 Apr 1; 161:258-64. Epub 2016 Feb 18.
- Review Alprazolam.[LiverTox: Clinical and Researc...]Review Alprazolam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Flurazepam.[LiverTox: Clinical and Researc...]Review Flurazepam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Temazepam.[LiverTox: Clinical and Researc...]Review Temazepam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Chlordiazepoxide - LiverToxChlordiazepoxide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...