NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Chlorambucil is an orally administered alkylating agent which is currently used in the therapy of chronic lymphocytic leukemia, Hodgkin and non-Hodgkin lymphomas, and rarely in severe autoimmune conditions including rheumatoid arthritis, uveitis and nephrotic syndrome. Chlorambucil therapy has been associated with low rates of serum enzyme elevations during therapy and to rare instances of acute, clinically apparent injury.
Background
Chlorambucil (klor am' bue sil) is an orally available, alkylating agent similar to mechlorethamine, cyclophosphamide and melphalan (nitrogen mustard-like). The alkylating agents act by causing modification and cross linking of DNA, thus inhibiting DNA, RNA and protein synthesis and causing cell death in rapidly dividing cells. Alkylating agents also have immunosuppressive activity, and chlorambucil has also been used in the therapy of autoimmune diseases and allograft rejection. Chlorambucil was approved for use in the United States in 1957. Current major indications include Hodgkin and non-Hodgkin lymphomas, chronic lymphocytic leukemia and lymphosarcoma. Chlorambucil has also been used for macroglobulinemia, polycythemia vera, rheumatoid arthritis, autoimmune uveitis and minimal change nephrotic syndrome, but its link to development of leukemia and secondary cancers has led to recommendations that its use be restricted to malignant conditions. Chlorambucil is available as Leukeran in tablets of 2 mg, and the usual dose ranges from 2 to 10 mg daily, often being given long term. Chlorambucil is generally well tolerated, and flexible dosing allows for dose adjustment to minimize side effects. Chlorambucil shares common side effects with other alkylating agents such as nausea, vomiting, diarrhea, alopecia, oral ulcers, bone marrow suppression, hypersensitivity reactions and rash. Long term therapy may be associated with an increased rate of acute myelocytic leukemia, interstitial pneumonitis and pulmonary fibrosis. Acute hypersensitivity reactions are rare but can include drug fever, angioedema, erythema multiforme, and Stevens Johnson syndrome.
Hepatotoxicity
Chlorambucil therapy is associated with a low rate of serum enzyme elevations, but these are generally mild and self limited, not requiring dose adjustment. Rare instances of clinically apparent acute liver injury attributed to chlorambucil have been reported. The onset of symptoms was within 2 to 6 weeks of starting chlorambucil and the typical enzyme pattern was cholestatic. Some cases have had features of hypersensitivity (rash, fever), and liver injury has recurred upon rechallenge. This form of liver injury is rare and resembles the idiosyncratic acute liver injury due to cyclophosphamide. Chlorambucil has not been linked specifically to sinusoidal obstruction syndrome, but it is not used in high doses in neoplastic disease or in conditioning regimens for hematopoietic cell transplantation, situations in which alkylating agents are commonly associated with this complication. Chlorambucil therapy has also been linked to hypersensitivity reactions and severe cutaneous adverse events such as Stevens Johnson syndrome and toxic epidermal necrolysis, both of which can be accompanied by serum enzyme elevations and hepatitis.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The idiosyncratic cholestatic liver injury associated with chlorambucil use is probably related to a hypersensitivity reaction to a hepatic metabolite of the drug, which is extensively metabolized by the liver.
Outcome and Management
Liver injury is rare after chlorambucil therapy. The severity of injury in reported cases has been mild-to-moderate and self limited in course. There have been no instances of acute liver failure, chronic hepatitis or vanishing bile duct syndrome definitively linked to chlorambucil therapy. In situations of acute liver injury after chlorambucil use, rechallenge should be avoided and there may be some degree of cross sensitivity to allergic reactions with other nitrogen mustard-like alkylating agents such as cyclophosphamide and melphalan.
Drug Class: Antineoplastic Agents, Alkylating Agents
CASE REPORT
Case 1. Cholestatic hepatitis attributed to chlorambucil.
[Modified from: Pichon N, Debette-Gratien M, Cessot F, Paraf F, Labrousse F, Sautereau D, Pillegand B. [Acute cholestatic hepatitis caused by chlorambucil]. Gastroenterol Clin Biol 2001; 25: 202-3. PubMed Citation]
A 77 year old woman with non-Hodgkin lymphoma developed pruritus and jaundice 6 weeks after starting oral chlorambucil (4 mg daily). She had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. Her other medications included sertraline which she had taken for 6 months. On examination, she had splenomegaly which was unchanged from before and new findings of jaundice and excoriations over the forearms. Laboratory testing showed a total serum bilirubin of 13.3 mg/dL (direct 11.8 mg/dL), accompanied by elevations in serum aminotransferase and alkaline phosphatase levels (Table). There was no eosinophilia and the prothrombin time was normal. Tests for hepatitis A, B and C were negative as were autoantibodies. Ultrasound and computerized tomography of the abdomen showed no evidence of biliary obstruction, which was further confirmed by magnetic resonance cholangio-pancreatography. A liver biopsy showed an intrahepatic cholestasis with prominence of eosinophils suggestive of drug induced liver injury. There was also mild congestion of sinusoids and changes of peliosis. There was no bile duct injury or loss. Both chlorambucil and sertraline were stopped and her symptoms and liver test abnormalities gradually improved. Sertaline was restarted and all laboratory results were normal 2 months later.
Key Points
| Medication: | Chlorambucil |
|---|---|
| Pattern: | Cholestatic (R=0.4) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 6 weeks |
| Recovery: | 2 months |
| Other medications: | Sertraline |
Laboratory Values
* Modified from Table 1.
Comment
A typical case of cholestatic hepatitis in which chlorambucil was the likely cause. Sertraline has also been associated with drug induced liver injury, but was unlikely the cause in this instance as shown by the negative rechallenge. The appearance of pruritus at the onset of illness and prolonged jaundice are typical of drug induced cholestatic hepatitis.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Chlorambucil – Leukeran®
DRUG CLASS
Antineoplastic Agents, Alkylating
Product labeling at DailyMed, National Library of Medicine, NIH
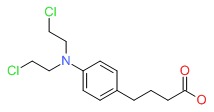
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Chlorambucil | 305-03-3 | C14-H19-Cl2-N-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 05 October 2017
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; mentions that chlorambucil has been linked to several reports of liver injury, which has usually been hepatocellular and occasionally severe).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; chlorambucil is not specifically discussed).
- Chabner BA, Bertino J, Clearly J, Ortiz T, Lane A, Supko JG, Ryan DP. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1677-730.(Textbook of pharmacology and therapeutics).
- Koler RD, Forsgren AL. Hepatotoxicity due to chlorambucil; report of a case. JAMA 1958; 167: 316-7. [PubMed: 13538708](36 year old man with chronic lymphocytic leukemia developed rash 2 weeks after starting chlorambucil, followed by nausea and jaundice [bilirubin 6.2], resolving within 6 weeks of stopping; rash and hepatomegaly recurred 3 days after restarting chlorambucil).
- Amromin GD, Deliman RM, Shanbrom E. Liver damage after chemotherapy for leukemia and lymphoma. Gastroenterology 1962; 42: 401-10. [PubMed: 13861044](Among 181 patients receiving chemotherapy for cancer, 22 [16%] developed jaundice, 10 being attributed to chemotherapy, 6 possibly due to chlorambucil with onset after 18-75 days with degenerative and cholestatic changes on biopsy).
- Morgano G, Patrone E, Muratore A. [Atypical aspects of leukoses. Venous thrombosis; jaundice]. Minerva Med 1969; 60: 1106-11. Italian. [PubMed: 5252723](69 year old man with chronic lymphocytic leukemia developed jaundice within a month of starting chlorambucil [bilirubin not given, ALT 340 U/L, Alk P 2 times ULN], resolving after stopping).
- Jick H, Walker AM, Porter J. Drug-induced liver disease. J Clin Pharmacol 1981; 21: 359-64. [PubMed: 7276230](Among 144 medical inpatients receiving chlorambucil, one [57 year old woman with hemolytic anemia] developed drug induced liver injury after 13 days of use; recovered after discontinuation; no specific details given).
- Renambot J, Aubry P, Menard M, Dano P, Brunetti G. [Hepatocellular carcinoma in an African after 10 years of treatment of Waldenstrom's disease with chlorambucil]. Nouv Presse Med 1982; 11: 2418. French. [PubMed: 6287413](47 year old Senegalese man with Waldenstrom macroglobulinemia treated with chlorambucil for 10 years developed hepatocellular carcinoma, but without cirrhosis and with negative tests for HBsAg [immunodiffusion]).
- Rollins BJ. Hepatic veno-occlusive disease. Am J Med 1986; 8: 297-306. [PubMed: 3526887](Review of the diagnosis, clinical course, histology and pathogenesis of veno-occlusive disease, now referred to as sinusoidal obstruction syndrome [SOS]).
- Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, et al. Veno-occlusive disease of the liver following bone marrow transplantation. Transplantation 1987; 4: 778-83. [PubMed: 3321587](Among 235 patients undergoing hematopoietic cell transplantation between 1982 and 1985, sinusoidal obstruction syndrome [SOS] developed in 52 [22%] of whom half died, making SOS the third most common cause of death in this population).
- Barone C, Cassano A, Astone A. Toxic epidermal necrolysis during chlorambucil therapy in chronic lymphocytic leukaemia. Eur J Cancer 1990; 26: 1262. [PubMed: 2150004](57 year old woman with CLL developed toxic epidermal necrosis after 18 days of chlorambucil therapy, and recurrence of rash and bullae within a few hours of restarting it; no mention of jaundice or liver test results).
- Aydogdu I, Ozcan C, Harputluoglu M, Karincaoglu Y, Turhan O, Ozcanu A. Severe adverse skin reaction to chlorambucil in a patient with chronic lymphocytic leukemia. Anticancer Drugs 1997; 8: 468-9. [PubMed: 9215610](60 year old man with CLL developed toxic epidermal necrolysis 20 days after starting chlorambucil, which resolved within 3 weeks of stopping; no mention of liver test results).
- Pichon N, Debette-Gratien M, Cessot F, Paraf F, Labrousse F, Sautereau D, Pillegand B. [Acute cholestatic hepatitis caused by chlorambucil]. Gastroenterol Clin Biol 2001; 25: 202-3. [PubMed: 11319447](77 year old woman with non-Hodgkin lymphoma developed pruritus and jaundice 6 weeks after starting chlorambucil [4 mg/day], [bilirubin 29.8 mg/dL, ALT 2 times ULN, Alk P 4.5 times ULN, no eosinophilia], resolving within 3 months of stopping: Case 1).
- Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood 1995; 85: 3005-20. [PubMed: 7756636](Review of hepatic veno-occlusive disease after bone marrow transplantation; usually presents with painful hepatomegaly, weight gain [fluid and ascites] and jaundice within 3 weeks of myeloablation with occlusion of central veins and sinusoids and extensive zone 3 [centrolobular] injury).
- Idasiak-Piechocka I, Oko A, Łochyńska-Bielecka K, Skrobańska B. Efficacy and safety of low-dose chlorambucil in nephrotic patients with idiopathic membranous nephropathy. Kidney Blood Press Res 2009; 32: 263-7. [PubMed: 19776643](Among 32 patients with membranous nephropathy treated with chlorambucil and corticosteroids, none had serious adverse events or hepatotoxicity).
- Knauf WU, Lissichkov T, Aldaoud A, Liberati A, Loscertales J, Herbrecht R, Juliusson G, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009; 27: 4378-84. [PubMed: 19652068](Among 312 patients with chronic lymphocytic leukemia treated with chlorambucil or bendamustine for up to 4 years, clinical responses were more common with bendamustine [68% vs 31%], while side effects were similar and there was no mention of ALT elevations or hepatotoxicity).
- Rosen AC, Balagula Y, Raisch DW, Garg V, Nardone B, Larsen N, Sorrell J, et al. Life-threatening dermatologic adverse events in oncology. Anticancer Drugs 2014; 25: 225-34. [PMC free article: PMC3890653] [PubMed: 24108082](In a systematic review of reports of Stevens Johnson Syndrome and toxic epidermal necrolysis due to antineoplastic agents from the literature and the MedWatch system, chlorambucil is listed as one of the more common implicated agents in serious cutaneous reactions, although most patients were taking other potentially causative medications).
- Hillmen P, Gribben JG, Follows GA, Milligan D, Sayala HA, Moreton P, Oscier DG, et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: Final analysis of an open-label phase II study. J Clin Oncol 2014; 32: 1236-41. [PMC free article: PMC4876343] [PubMed: 24638012](Among 100 patients with CLL treated with rituximab and chlorambucil in 6 28-day cycles, the overall response rate was 84% and adverse events were mostly hematologic; there were no serious adverse hepatic events and no mention of ALT elevations).
- Hillmen P, Robak T, Janssens A, Babu KG, Kloczko J, Grosicki S, Doubek M, et al; COMPLEMENT 1 Study Investigators. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet 2015; 385 (9980): 1873-83. [PubMed: 25882396](Among 447 patients with CLL treated with chlorambucil with or without ofatumumab [anti-CD20 monoclonal antibody], progression free survival was greater with ofatumumab [22 vs 13 months] as were overall adverse event rates, but there were no liver-related serious adverse events or deaths).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 cases [5%] were attributed to antineoplastic agents including several to alkylating agents, but none to chlorambucil).
- Lepretre S, Dartigeas C, Feugier P, Marty M, Salles G. Systematic review of the recent evidence for the efficacy and safety of chlorambucil in the treatment of B-cell malignancies. Leuk Lymphoma 2016; 57: 852-65. [PubMed: 26308278](In a systematic review of 9 controlled trials of chlorambucil for B-cell malignancies, its adverse event rate was considered low, the most common events being nausea, neutropenia, and lymphopenia; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Bendamustine.[LiverTox: Clinical and Researc...]Review Bendamustine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- CHLORAMBUCIL THERAPY FOR LYMPHOMAS AND CHRONIC LYMPHOCYTIC LEUKEMIA.[JAMA. 1965]CHLORAMBUCIL THERAPY FOR LYMPHOMAS AND CHRONIC LYMPHOCYTIC LEUKEMIA.EZDINLI EZ, STUTZMAN L. JAMA. 1965 Feb 8; 191:444-50.
- Review Chlorambucil-induced seizures.[Cancer. 1997]Review Chlorambucil-induced seizures.Salloum E, Khan KK, Cooper DL. Cancer. 1997 Mar 1; 79(5):1009-13.
- Review Bendamustine for the treatment of chronic lymphocytic leukemia and rituximab-refractory, indolent B-cell non-Hodgkin lymphoma.[Clin Ther. 2009]Review Bendamustine for the treatment of chronic lymphocytic leukemia and rituximab-refractory, indolent B-cell non-Hodgkin lymphoma.Dennie TW, Kolesar JM. Clin Ther. 2009; 31 Pt 2:2290-311.
- Review Altretamine.[LiverTox: Clinical and Researc...]Review Altretamine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Chlorambucil - LiverToxChlorambucil - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...