NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Carmustine (BCNU) is a parenterally administered alkylating agent used alone and in combination with other antineoplastic agents in the treatment of several forms of cancer including leukemias, lymphomas, and breast, testicular, ovarian, gastric and pancreatic cancer. Carmustine therapy is associated with minor transient serum enzyme elevations and has been linked to cases of acute liver injury including cholestatic hepatitis and acute veno-occlusive disease.
Background
Carmustine (kar mus' teen), which is also known as BCNU, is a nitrosourea that acts as an alkylating agent and is used in the therapy of several forms of leukemia, lymphoma and solid organ cancer. Like cyclophosphamide, carmustine requires activation in the liver to form its active intermediates which act by modifying and cross linking purine bases in DNA, thus inhibiting DNA, RNA and protein synthesis and leading to cell death in rapidly dividing cells. Carmustine also forms adducts with intracellular proteins. Carmustine was approved for use in the United States in 1977, and its current uses include treatment of breast, gastric, liver, pancreatic, lung, brain, ovarian and testicular cancer, malignant melanoma, Hodgkin and non-Hodgkin lymphoma and multiple myeloma. Carmustine is given intravenously and is available in liquid formulations (100 mg vials) in generic forms and under the trade names Gliadel or BiCNU. Recommended doses vary by age, body weight and malignant condition. Carmustine is often given in combination with other antineoplastic agents or alone in cycles every 6 to 8 weeks. It is also available formulated in a wafer containing 7.7 mg of carmustine (Gliadel), which can be inserted in a surgical space such as the brain after resection of a high grade malignant glioma. The toxicity of carmustine is similar to other alkylating agents. Common side effects include alopecia, nausea, vomiting, diarrhea, gastrointestinal upset, nephrotoxicity, oral ulcers and bone marrow suppression.
Hepatotoxicity
Mild and transient elevations in serum aminotransferase levels are found in up to 25% of patients treated with carmustine. Because carmustine is typically given in combination with other agents, its role in causing these serum enzyme elevations is not always clear. The abnormalities are generally transient, do not cause symptoms and do not require dose modification. Clinically apparent liver injury from carmustine has been limited to a small number of cases of cholestatic hepatitis and more frequent instances of sinusoidal obstruction syndrome, reported mostly with its use in high doses or as a conditioning agent in preparation for hematopoietic cell transplantation. The onset of sinusoidal obstruction syndrome is usually within two to three weeks of the myeloablation and is characterized by a sudden onset of abdominal pain, weight gain, ascites, marked increase in serum aminotransferase levels (and LDH), and subsequent jaundice and hepatic dysfunction. The severity of sinusoidal obstruction syndrome varies from a transient, self-limited injury to acute liver failure. The diagnosis of sinusoidal obstruction syndrome is usually based on clinical features of tenderness and enlargement of the liver, weight gain, ascites and jaundice occurring within 3 weeks of chemotherapy. Liver biopsy is diagnostic but often contraindicated, because of severe thrombocytopenia after hematopoietic cell transplantation.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury, particularly when used for myeloablation).
Mechanism of Injury
Most instances of hepatotoxicity from carmustine appear dose related and probably due to direct cytotoxicity. The sinusoidal obstruction syndrome induced by alkylating agents is probably related to their toxic effect on sinusoidal cells in the liver, causing their necrosis and release into the sinusoids, with subsequent obstruction and obliteration of hepatic veins. Carmustine is extensively metabolized by the hepatic cytochrome P450 system.
Outcome and Management
The severity of liver injury from carmustine ranges from mild elevations in liver enzymes, to a self limited cholestatic hepatitis to massive, fatal hepatic necrosis due to sinusoidal obstruction syndrome. There is currently no specific therapy for veno-occlusive disease other than supportive management and avoidance of further damage. Rechallenge should be avoided.
Drug Class: Antineoplastic Agents, Alkylating Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Carmustine – BiCNU, Gliadel®
DRUG CLASS
Antineoplastic Agents, Alkylating Agents
Product labeling at DailyMed, National Library of Medicine, NIH
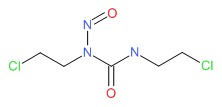
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Carmustine | 154-93-8 | C5-H9-Cl2-N3-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 17 January 2017
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; mentions that carmustine has been linked to cases of hepatocellular injury with jaundice).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 541-68.(Review of hepatotoxicity of cancer chemotherapeutic agents lists carmustine as having liver test elevations in up to 25% of patients and being linked to occasional case report of injury and even fatalities).
- Chabner BA, Bertino J, Clearly J, Ortiz T, Lane A, Supko JG, Ryan DP. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1677-730.(Textbook of pharmacology and therapeutics).
- De Vita VT, Carbone PP, Owens AH Jr, Gold GL, Krant MJ, Edmonson J. Clinical trials with 1,3-bis(2-chloroethyl)-1-nitrosourea, NSC-409962. Cancer Res 1965; 25: 1876-81. [PubMed: 5858571](Preliminary studies of BCNU in 144 patients with malignancies using different dose schedules; AST elevations occurred in 15% after 13-63 days and were self-limited in all; liver toxicity may have contributed to the death of one patient).
- Hansen HH, Selawry OS, Muggia FM, Walker MD. Clinical studies with 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (NSC 79037). Cancer Res 1971; 31: 223-7. [PubMed: 4926242](Preliminary studies of CCNU in 40 patients with malignancies; there was no consistent hepatic dysfunction; AST elevations occurred in one patient).
- Young RC, De Vita VT, Serpick AA, Cancellos GP. Treatment of advanced Hodgkin's disease with [1,3-bis(2-chloroethyl)-1-nitrosourea] BCNU. N Engl J Med 1967; 285: 475-9. [PubMed: 5558887](Among 45 patients with refractory Hodgkin disease treated with carmustine, 21 [47%] achieved a remission; mentions that one patient who died had liver toxicity).
- Walker MD, Hurwitz BS. BCNU(1,2-bis(2-chloroethyl)-1-nitrosourea; NSC-409962) in the treatment of malignant brain tumor- a preliminary report. Cancer Chemother Rep 1970; 54: 263-71. [PubMed: 4946011](Pilot study of carmustine in 27 patients with brain tumors mentions that there were mild elevations in AST and Alk P in a few patients, but that these rapidly returned to normal).
- Lokich JJ, Drum DE, Kaplan W. Hepatic toxicity of nitrosourea analogues. Clin Pharmacol Ther 1974; 16: 363-7. [PubMed: 4852251](2 cases: 70 year old man with metastatic colon cancer and cirrhosis developed jaundice 6 weeks after a 5 day course of carmustine and streptozotocin [bilirubin 6.2 mg/dL], but no other information given; 66 year old man with pancreatic cancer developed jaundice 2 weeks after carmustine and streptozotocin [bilirubin 3.5 mg/dL, AST 30 U/L, Alk P 135 U/L], with recovery after stopping therapy).
- Lessner HE, Vogler WR. Toxicity study of BCNU (NSC-409965) given orally. Cancer Chemother Rep 1974; 58: 407-11. [PubMed: 4601671](Pilot study of carmustine alone in various oral doses in 104 patients with cancer; two patients developed liver toxicity, one with jaundice and elevated Alk P, but relationship to drug was considered uncertain).
- McIntyre RE, Magidson JG, Austin GE, Gale RP. Fatal veno-occlusive disease of the liver following high dose 1,3-bis(2-chloroethyl)-1-nitrosurea (BCNU) and autologus bone marrow transplantation. Am J Clin Pathol 1981; 75: 614-6. [PubMed: 7013473](22 year old woman with lymphoma developed fatal sinusoidal obstruction syndrome arising 18 days after bone marrow transplantation, preceded by myeloablation using high dose carmustine alone).
- Brandes AA, Tosoni A, Amistà P, Nicolardi L, Grosso D, Berti F, Ermani M. How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial. Neurology 2004: 63: 1281-4. [PubMed: 15477552](Among 40 patients with recurrent glioblastoma treated with 100 cycles of carmustine, hepatotoxicity [grade 2 or 3] occurred in 4 patients [10%], abnormalities resolving on average within 2 months of stopping).
- Wolff SN. High-dose carmustine and high-dose etoposide: a treatment regimen resulting in enhanced hepatic toxicity. Cancer Treat Rep 1986; 70: 1464-5. [PubMed: 3791261](Pilot study of high dose carmustine and etoposide in 4 patients with astrocytoma; 3 developed hepatotoxicity with ascites arising 1-2 months after starting therapy which was fatal in 2).
- Phillips GL, Fay JW, Herzig GP, Herzig RH, Weiner RS, Wolff SN, Lazarus HM, et al. Intensive 1,3-bis(2-chloroethyl)-1-nitrosourea(BCNU), NSC #4366650 and cryopreserved autologous marrow transplantation for refractory cancer. A phase I-II study. Cancer 1983; 52: 1792-802. [PubMed: 6354414](Among 93 patients with refractory cancer treated with escalating doses of carmustine and hematopoietic cell transplantation, hepatotoxicity occurred in 29 [31%], which was severe in 10 and fatal in 8 [9%], marked by cholestasis and hepatic failure with centrolobular hepatic necrosis and sinusoidal obstruction syndrome (SOS) in some cases, mostly with higher doses).
- Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, et al. Veno-occlusive disease of the liver following bone marrow transplantation. Transplantation 1987; 4: 778-83. [PubMed: 3321587](Among 235 patients undergoing hematopoietic cell transplantation between 1982 and 1985, sinusoidal obstruction syndrome [SOS] developed in 52 [22%] of whom half died, making SOS the third most common cause of death in this population).
- Ahmed T, Ciavarella D, Feldman E, Ascensao J, Hussain F, Engelking C, Gingrich S, et al. High-dose, potentially myeloablative chemotherapy and autologous bone marrow transplantation for patients with advanced Hodgkin's disease. Leukemia 1989; 3: 19-22. [PubMed: 2642573](Among 23 patients with refractory Hodgkin disease treated with high doses of carmustine, etoposide and cyclophosphamide and autologous hematopoietic cell transplantation, 39% had liver test abnormalities and one died of hepatotoxicity; no details given).
- Jones RB, Shpall EJ, Ross M, Coniglio D, Affronti ML, Peters WP. High-dose carboplatin, cyclophosphamide, and BCNU with autologous bone marrow support: excessive hepatic toxicity. Cancer Chemother Pharmacol 1990; 26: 155-6. [PubMed: 2189592](Among 4 patients given high dose carboplatin, cyclophosphamide and carmustine followed by hematopoietic cell transplantation for malignant melanoma, 3 developed severe SOS and two died and the fourth became jaundiced).
- Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood 1995; 85: 3005-20. [PubMed: 7756636](Review of sinusoidal obstruction syndrome after hematopoietic cell transplantation; usually presents with painful hepatomegaly, weight gain [fluid and ascites] and jaundice within 3 weeks of myeloablation, with occlusion of central veins and sinusoids and extensive zone 3 [centrolobular] injury).
- Papadakis V, Dunkel IJ, Cramer LD, Kramer E, Papadopoulos E, Goldman S, Packer RJ, et al. High-dose carmustine, thiotepa and etoposide followed by autologous bone marrow rescue for the treatment of high risk central nervous system tumors. Bone Marrow Transplant 2000; 26: 153-60. [PubMed: 10918425](Among 42 patients with brain tumors who were treated with high dose carmustine, thiotepa and etoposide and autologous hematopoietic cell transplantation, 2 developed sinusoidal obstruction syndrome and 5 had transient elevations in ALT levels).
- DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome. Semin Liver Dis 2002; 22: 27-41. [PubMed: 11928077](Review of clinical features, pathology, etiology, prevention and treatment of sinusoidal obstruction syndrome [SOS], a better term for this condition than veno-occlusive disease; first described in association with exposure to phytotoxins [pyrrolizidine alkaloids], the most common cause now is cancer chemotherapy and particularly myeloablative conditioning regimens in preparation for hematopoietic cell transplantation).
- McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology 2010; 51: 1450-60. [PMC free article: PMC2914093] [PubMed: 20373370](Review of liver complications of bone marrow [hematopoietic cell] transplantation, which have become less frequent with better understanding of their causes and means of prevention; the rate of SOS has decreased because of avoidance of more aggressive ablative therapies [total body irradiation and high doses of cyclophosphamide] and better understanding of pharmacokinetics of the alkylating agents).
- Review Ifosfamide.[LiverTox: Clinical and Researc...]Review Ifosfamide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Sensitization of pancreatic tumor xenografts to carmustine and temozolomide by inactivation of their O6-Methylguanine-DNA methyltransferase with O6-benzylguanine or O6-benzyl-2'-deoxyguanosine.[Clin Cancer Res. 2003]Sensitization of pancreatic tumor xenografts to carmustine and temozolomide by inactivation of their O6-Methylguanine-DNA methyltransferase with O6-benzylguanine or O6-benzyl-2'-deoxyguanosine.Kokkinakis DM, Ahmed MM, Chendil D, Moschel RC, Pegg AE. Clin Cancer Res. 2003 Sep 1; 9(10 Pt 1):3801-7.
- Combination of intracranial temozolomide with intracranial carmustine improves survival when compared with either treatment alone in a rodent glioma model.[Neurosurgery. 2010]Combination of intracranial temozolomide with intracranial carmustine improves survival when compared with either treatment alone in a rodent glioma model.Recinos VR, Tyler BM, Bekelis K, Sunshine SB, Vellimana A, Li KW, Brem H. Neurosurgery. 2010 Mar; 66(3):530-7; discussion 537.
- Acute lung injury following treatment with high-dose cyclophosphamide, cisplatin, and carmustine: pharmacodynamic evaluation of carmustine.[J Natl Cancer Inst. 1993]Acute lung injury following treatment with high-dose cyclophosphamide, cisplatin, and carmustine: pharmacodynamic evaluation of carmustine.Jones RB, Matthes S, Shpall EJ, Fisher JH, Stemmer SM, Dufton C, Stephens JK, Bearman SI. J Natl Cancer Inst. 1993 Apr 21; 85(8):640-7.
- Review Cyclophosphamide.[LiverTox: Clinical and Researc...]Review Cyclophosphamide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Carmustine - LiverToxCarmustine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...