NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Cabotegravir is an antiviral agent that inhibits the human immunodeficiency virus (HIV) integrase and is used in combination with rilpivirine, a non-nucleoside HIV reverse transcription inhibitor, in the treatment of HIV infection and the acquired immunodeficiency syndrome (AIDS). The fixed combination of cabotegravir and rilpivirine is typically given intramuscularly once monthly and has been linked to a low rate of serum aminotransferase elevations during therapy and to rare episodes of acute, clinically apparent liver injury.
Background
Cabotegravir (ka” boe teg’ ra vir) is an antiretroviral drug that targets the HIV integrase, one of the three critical viral enzymes involved in HIV replication. Cabotegravir binds to the catalytic site of the HIV integrase, preventing the strand transfer activity and integration of the provirus into the host genome. Cabotegravir has both in vitro and in vivo activity against HIV and was developed as both oral and parenterally administered forms. In several randomized controlled trials, injections of a long acting formulation of cabotegravir in a fixed combination with a long acting rilpivirine, a non-nucleoside HIV reverse transcriptase inhibitor, at 4 or 8 week intervals was found to effectively replace stable, effective daily oral regimens for HIV infection. Cabotegravir was approved as therapy of HIV infection in the United States in 2021, but only in combination with rilpivirine in both tablet (oral) forms as a 4 week lead-in therapy to parenteral therapy with solutions of long acting cabotegravir and rilpivirine given by intramuscular injections every 4 or 8 weeks for long term maintenance therapy. The fixed combination of cabotegravir and rilpivirine was initially indicated only for patients with HIV infection on an oral antiviral regimen that effectively suppressed viral levels to less than 50 copies/mL. The oral forms of cabotegravir and rilpivirine were approved only as lead-in to starting the monthly parenteral administration (or as temporary therapy of patients who miss a monthly injection). The long acting formulation of cabotegravir was approved for use as monotherapy in 2022 as a means of preventing HIV infection in high risk individuals. Cabotegravir is available in tablets of 30 mg under the brand name Vocabria to be used in combination with rilpivirine in tablets of 25 mg for one month before initiating parenteral injections. Cabotegravir and rilpivirine are also available in solution for intramuscular injection as a fixed combination of 400 and 600 mg for every 4 week administration and 600 and 900 mg for every 8 week administration under the brand name Cabenuva. Clinical trials have more recently shown that the combination of cabotegravir and rilpivirine can be used as an initial means of treating HIV infection without an oral lead-in phase. Finally, the long acting formulation of cabotegravir in solution is available separately for prevention of HIV infection in high risk individuals under the brand name Apretude. Common side effects of cabotegravir include headache, nausea, insomnia, vivid dreams, and anxiety, as well as injection site reactions with parenteral regimens. Less common but potentially severe adverse events include hypersensitivity reactions (fever, rash, angioedema, conjunctivitis, blisters and oral ulcers, DRESS syndrome), hepatotoxicity, and depression.
Hepatotoxicity
In large clinical trials, switching antiretroviral therapy of HIV infection to the combination of parenteral injections of long acting cabotegravir and rilpivirine was associated with alanine aminotransferase (ALT) elevations in up to 7% of patients, but levels above 5 times the upper limit of normal (ULN) arose in only 1% to 2% of subjects. The elevations were typically transient, asymptomatic, and rarely required dose modification or discontinuation. While clinically apparent liver injury with jaundice was reported to occur in early studies, there were no such cases in the large, preregistration trials of long acting, parenteral cabotegravir and rilpivirine used for treatment of HIV infection or in the large trials of long acting cabotegravir used as prophylaxis against acquiring HIV infection. The use of injections every 4 or 8 weeks has been found to improve compliance and to be preferable to daily oral therapy by many patients requiring long term antiretroviral therapy. Since approval of cabotegravir for use as maintenance therapy and for prophylaxis against HIV infection, there have been no case reports of clinically apparent hepatotoxicity associated with its use.
Interestingly, in the large preregistration trials of parenteral cabotegravir and rilpivirine as replacement therapy of HIV infection, cases of acute hepatitis A, B and C were reported as adverse events, occurring largely in patients who had been switched to the parenteral regimen compared to control subjects who were continued on oral agents. The reasons for these differences were unclear. Importantly, neither cabotegravir or rilpivirine have activity against hepatitis B virus (HBV), and one possibility has been that reactivation of hepatitis B may occur after withdrawal of oral antiretroviral agents that have activity against HBV, such as tenofovir, emtricitabine, and lamivudine. For this reason, patients starting long- acting parenteral regimens of cabotegravir and rilpivirine should be screened for hepatitis B virus markers and potential risks of stopping agents with activity against HBV should be considered. In addition, persons with HIV without protective antibodies to HAV and HBV should be offered hepatitis A and B virus vaccination.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury).
Mechanism of Injury
Cabotegravir is metabolized by the liver and undergoes glucuronidation as a part of its metabolic clearance. It does not have an effect on the CYP 450 system. Concomitant use of potent inducers of UGT1A can result in lower plasma levels which can predispose to virologic failure and development of antiviral resistance.
Outcome and Management
Serum enzyme elevations during combination therapy with cabotegravir and rilpivirine are not uncommon but are generally transient, mild-to-moderate in severity, and often attributable to other causes. Nevertheless, aminotransferase elevations above 5 times the ULN, if confirmed, warrant dose interruption and evaluation for other causes of acute liver injury. Because cabotegravir is usually given in combination with rilpivirine, it is difficult to attribute the injury to a single agent, and the combination generally needs to be discontinued if the injury is persistent or associated with symptoms or jaundice. HIV integrase inhibitors have rarely been implicated in causing hepatotoxicity and there is no information about the cross sensitivity to liver injury among the various HIV integrase inhibitors.
Drug Class: Antiviral Agents
Other Drugs in the Subclass, Integrase Strand Transfer Inhibitors: Bictegravir, Dolutegravir, Elvitegravir, Raltegravir
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cabotegravir – Cabenuva®, Apretude®, Vocabria®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Cabotegravir | 1051375-10-0 | C19-H17-F2-N3-O5 |

|
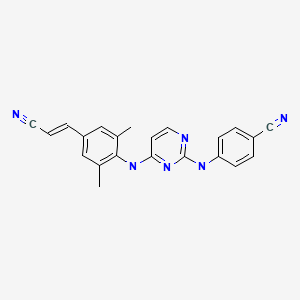
| Rilpivirine | 500287-72-9 | C22-H18-N6 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 17 June 2023
Abbreviations: HIV, human immunodeficiency virus; im, intramuscular.
- Núñez M. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 505-18.(Review of hepatotoxicity of antiviral agents including raltegravir but not cabotegravir).
- Flexner C. Antiretroviral agents and treatment of HIV infection. In, Brunton LL, Hillal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1137-57.(Textbook of pharmacology and therapeutics).
- https://hivinfo
.nih.gov /hiv-source/medical-practice-guidelines /hiv-treatment-guidelines . (Clinical guidelines on the use of antiretroviral agents in HIV-1 infected adults, adolescents and children). - FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2021/212887Orig1s000,212888Orig1s000IntegratedR .pdf. (FDA website with product labels and the integrated review of the data submitted in support of its approval, mentions that liver injury suspected to be drug induced arose in early phase 2 trials of cabotegravir but there were no cases in the large-scale, phase 3 registration trials; however, there were multiple cases of acute hepatitis A, B and C that were not identified in control groups, some of which may have related to withdrawal of antiretroviral agents with activity against HBV since cabotegravir and rilpivirine have no activity against hepatitis B, furthermore patients with HIV infection should be given both hepatitis A and B vaccines as these are preventable diseases). - Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 were due to antiretroviral agents, but none were attributed to cabotegravir or other HIV integrase inhibitors).
- Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, Eron JJ, Yazdanpanah Y, Podzamczer D, Lutz T, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510. [PubMed: 28750935](Among 309 treatment naïve adults with HIV infection who were treated with oral cabotegravir [30 mg once daily], lamivudine and abacavir for 20 weeks, 286 had a virologic response [HIV RNA <50 copies/mL] and were randomized to continue oral therapy or switch to long acting intramuscular [im] cabotegravir and rilpivirine every 4 weeks [400 mg and 600 mg] or every 8 weeks [600 mg or 900 mg] and followed for up to 96 weeks, during which the 3 treatment arms had similar rates of virologic response maintenance and similar rates of adverse events except for injection site reactions with the im regimens; ALT elevations above 5 times ULN arose in 3% on im regimens and 5% on oral regimens mostly due to hepatitis C infection, but two patients on the oral regimen were judged to have probable drug induced liver injury which resolved with stopping therapy [no details provided]).
- Kolakowska A, Maresca AF, Collins IJ, Cailhol J. Update on adverse effects of HIV integrase inhibitors. Curr Treat Options Infect Dis. 2019;11:372–387. [PMC free article: PMC7758219] [PubMed: 33380904](Review of the adverse events with HIV integrase inhibitors including dolute-, ralte-, bicte- and elvite-gravir mentions that there is little evidence that this class of antiretrovirals causes elevations in serum aminotransferase or alkaline phosphatase levels, but that data on the safety and efficacy of cabotegravir are promising but still incomplete).
- Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, Latiff G, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382:1112–1123. [PubMed: 32130809](Among 616 adults with HIV infection and maintained suppression of HIV RNA [less than 50 copies/mL] on oral antiretroviral therapy, who were either continued on oral therapy or switched to monthly injections of long acting cabotegravir and rilpivirine, effective suppression was maintained in 95.5% on oral therapy vs 92.5% or long acting parenteral therapy, while adverse event rates were similar except for injection site reactions [none vs 81%], while acute viral hepatitis [A, B and C] was identified as an adverse event in 1 case on oral and 4 on parenteral therapy [0.3% vs 1.3%]).
- Orkin C, Arasteh K, Górgolas Hernández-Mora M, Pokrovsky V, Overton ET, Girard PM, Oka S, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382:1124–1135. [PubMed: 32130806](Among 566 adults with previously untreated HIV infection with a virologic response [HIV RNA less than 50 copies/mL] to a 20-week induction regimen of dolutegravir, abacavir, and lamivudine who were then continued on oral therapy or switched to once-monthly injections with long acting cabotegravir and rilpivirine, maintenance of a virologic response was similar in the two groups [93.3% vs 93.6%] while adverse event rates were similar except for injection site reactions [none vs 80%] and acute hepatitis A, B, or C [1 vs 7 cases: 0.3% vs 2.5%]).
- Currier JS. Monthly injectable antiretroviral therapy – version 1.0 of a new treatment approach. N Engl J Med. 2020;382:1164–1165. [PubMed: 32130808](Editorial accompanying publications of Swindells and Orkin [2020] discussing the promise of a once monthly or bimonthly therapy for HIV infection).
- Cabotegravir/rilpivirine (Cabenuva) for HIV-1 infection. Med Lett Drugs Ther. 2021;63(1625):81–83. [PubMed: 34180282](Concise review of the mechanism of action, clinical efficacy, safety, and costs of the fixed combination of cabotegravir and rilpivirine given orally for one month and then as monthly injections for long term management of HIV infection).
- Landovitz RJ, Donnell D, Clement ME, Hanscom B, Cottle L, Coelho L, Cabello R, et al. HPTN 083 Study Team. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385:595–608. [PMC free article: PMC8448593] [PubMed: 34379922](Among 4566 adults at high risk of acquiring HIV infection given prophylaxis with oral tenofovir and emtricitabine daily or intramuscular cabotegravir [600 mg a baseline, week 4 and 9 and then every 8 weeks], incident HIV arose in 39 on oral prophylaxis [1.22 per 100-person years] vs 13 on cabotegravir infections [0.41 per 100-person years], while both total and serious adverse event rates were similar in the two groups with ALT elevations in 8.4% vs 7.0% which were above 3 times ULN in 1.0% vs 1.2%).
- Delany-Moretlwe S, Hughes JP, Bock P, Ouma SG, Hunidzarira P, Kalonji D, Kayange N, et al. HPTN 084 study group. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet. 2022;399(10337):1779–1789. [PMC free article: PMC9077443] [PubMed: 35378077](Among 3224 sexually active, Subsaharan African women, ages 18 to 45, treated with oral tenofovir and emtricitabine daily or intramuscular cabotegravir [600 mg at baseline, week 4 and every 8 weeks thereafter], incident HIV arose in 36 on oral prophylaxis vs 4 on injected cabotegravir [1.85 vs 0.2 per 100 person-years], while adverse events, except for injection site reactions, were similar in the two groups; 2 subjects on oral prophylaxis but none on cabotegravir developed serious hepatic adverse events).
- Cabotegravir (Apretude) for HIV-1 pre-exposure prophylaxis. Med Lett Drugs Ther. 2022;64(1644):29–31. [PubMed: 35171897](Concise review of the mechanism of action, efficacy, side effects, and costs of long acting, parenterally administered cabotegravir shortly after its approval an preventive therapy of HIV infection, mentions that hypersensitivity reactions, hepatotoxicity, and depressive disorders have been reported with cabotegravir therapy).
- Sutton KC, De Vente J, Leblanc R, Dejesus E, Smith G, Mills A, Baril JG, et al. Long-term efficacy, safety, and durability of cabotegravir and rilpivirine as 2-drug oral maintenance therapy after 6 years of study. Open Forum Infect Dis. 2022;9:ofac067. [PMC free article: PMC8946678] [PubMed: 35350172](Among 160 patients enrolled in a long-term extension study of long acting cabotegravir and rilpivirine injections, adverse events included injection site reactions, headaches, nausea, depression, and vivid dreams and serious adverse events were uncommon, one patient developing “increased liver function test”).
- Nachega JB, Scarsi KK, Gandhi M, Scott RK, Mofenson LM, Archary M, Nachman S, et al. Long-acting antiretrovirals and HIV treatment adherence. Lancet HIV. 2023;10:e332–e342. [PubMed: 37062293](Review of the availability, efficacy, safety, advantages and disadvantages of long-acting antivirals used for therapy and prevention of HIV including cabotegravir and rilpivirine; no mention of ALT elevations or hepatotoxicity).
- Overton ET, Richmond G, Rizzardini G, Thalme A, Girard PM, Wong A, Porteiro N, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with human immunodeficiency virus 1 type 1 infection: 152-week results from ATLAS-2M, a randomized, open-label, phase 3b, noninferiority study. Clin Infect Dis. 2023;76:1646–1654. [PMC free article: PMC10156123] [PubMed: 36660819](Among 1054 adults with HIV infection treated with injections of long acting cabotegravir and rilpivirine for up to three years, maintenance of HIV RNA suppression to less than 50 copies/mL was similar with every 4- vs 8-weekly administration [86% vs 87% at 3 years] with similar adverse event rates, ALT elevations above 3 times ULN arose in 6 subjects, 4 transiently and 2 requiring discontinuation of therapy - one being due to pancreatic cancer and one to chronic hepatitis C and alcohol abuse).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial.[Lancet Infect Dis. 2015]Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial.Margolis DA, Brinson CC, Smith GHR, de Vente J, Hagins DP, Eron JJ, Griffith SK, Clair MHS, Stevens MC, Williams PE, et al. Lancet Infect Dis. 2015 Oct; 15(10):1145-1155. Epub 2015 Jul 19.
- Cabotegravir: a novel HIV integrase inhibitor combined with rilpivirine as the first long-acting injectable program for the treatment of HIV infection.[Drugs Today (Barc). 2022]Cabotegravir: a novel HIV integrase inhibitor combined with rilpivirine as the first long-acting injectable program for the treatment of HIV infection.Zeuli JD, Rivera CG, Smith BL, Otto A, Temesgen Z. Drugs Today (Barc). 2022 Dec; 58(12):555-576.
- The potential role of long-acting injectable cabotegravir-rilpivirine in the treatment of HIV in sub-Saharan Africa: a modelling analysis.[Lancet Glob Health. 2021]The potential role of long-acting injectable cabotegravir-rilpivirine in the treatment of HIV in sub-Saharan Africa: a modelling analysis.Phillips AN, Bansi-Matharu L, Cambiano V, Ehrenkranz P, Serenata C, Venter F, Pett S, Flexner C, Jahn A, Revill P, et al. Lancet Glob Health. 2021 May; 9(5):e620-e627. Epub 2021 Mar 23.
- Review Cabotegravir/Rilpivirine: the last FDA-approved drug to treat HIV.[Expert Rev Anti Infect Ther. 2...]Review Cabotegravir/Rilpivirine: the last FDA-approved drug to treat HIV.Taki E, Soleimani F, Asadi A, Ghahramanpour H, Namvar A, Heidary M. Expert Rev Anti Infect Ther. 2022 Aug; 20(8):1135-1147. Epub 2022 Jun 13.
- Review A literature review of the patent application publications on cabotegravir - an HIV integrase strand transfer inhibitor.[Expert Opin Ther Pat. 2020]Review A literature review of the patent application publications on cabotegravir - an HIV integrase strand transfer inhibitor.Kovač L, Časar Z. Expert Opin Ther Pat. 2020 Mar; 30(3):195-208. Epub 2020 Jan 27.
- Cabotegravir - LiverToxCabotegravir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...