NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Bictegravir is a human immunodeficiency virus (HIV) integrase strand transfer inhibitor, the fourth in this class of agents that target the viral integrase. Bictegravir is used only in combination with other antiretroviral agents in the treatment of HIV infection and it has had limited use. Bictegravir is associated with a low rate of serum aminotransferase elevations during therapy, but has not been linked to instances of acute, clinically apparent liver injury.
Background
Bictegravir (bic teg' ra vir) is a relatively new antiretroviral drug that targets the HIV integrase, one of the three viral enzymes involved in replication. Bictegravir blocks the binding site of the HIV integrase and prevents the strand transfer activity and integration of the provirus into the host genome. Bictegravir has both in vitro and in vivo activity against HIV, and several randomized controlled trials have shown that it in combination with other antiretroviral agents can cause significant declines in HIV RNA levels and rises in peripheral CD4 T cell counts, demonstrating non-inferiority to established combination antiretroviral regimens of proven efficacy. Bictegravir (50 mg) in a fixed combination with emtricitabine (200 mg) and tenofovir alafenamide (25 mg) under the brand name Biktarvy was approved for use in HIV infection in the United States in 2018 and is currently used in an increasing proportion of antiretroviral regimens. The recommended dose is one tablet daily. Unlike the other integrase strand transfer inhibitors, bictegravir has not been marketed by itself to allow combination with other antiretroviral agents. Combination of Biktarvy with other antiretroviral agents is not recommended nor its use in patients with severe renal or liver dysfunction. While generally well tolerated, bictegravir therapy may be accompanied by diarrhea, headache, nausea and fever.
Hepatotoxicity
In large clinical trials, therapy with bictegravir combined with emtricitabine and tenofovir alafenamide was associated with alanine aminotransferase (ALT) elevations (above 1.5 times ULN) in 11% patients, but these rates were similar to those in comparator groups (12% to 15%) receiving matched background optimized antiretroviral therapy without bictegravir. Elevations above 5 times ULN occurred in only 1.4% of bictegravir vs 0.9% to 1.3% of control comparator arm subjects. The elevations were not associated with clinical symptoms and generally did not require dose modification. In addition, there were no instances of acute hepatocellular liver injury with jaundice. The product label for bictegravir mentions acute exacerbations of hepatitis B and hepatic failure as potential adverse reactions when bictegravir with emtricitabine and tenofovir is discontinued. This adverse reaction can occur upon discontinuation of any antiretroviral regimen with concurrent activity against HBV and represents the effects of tenofovir and emtricitabine. Nevertheless, since its approval and its more widescale use, there have been no published reports of clinically apparent cases of liver injury or exacerbation of hepatitis B convincingly attributed to bictegravir.
Likelihood score: E* (unproven but suspected potential cause of liver injury).
Mechanism of Injury
The mechanism by which bictegravir might cause liver injury is not known. Bictegravir is metabolized by the liver, largely by glucuronidation with subsequent urinary clearance. Bictegravir has little or no effect on microsomal cytochrome P450 enzymes. Reconstitution of immune reactivity by antiretroviral therapy in patients with an underlying chronic hepatitis B or C can be associated with a transient flare of hepatitis. Discontinuation of antiretroviral therapy with concurrent activity against HBV can cause severe exacerbations of hepatitis B.
Outcome and Management
If bictegravir hepatotoxicity occurs, it must be rare. Bictegravir has not been linked to cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome. Patients receiving antiviral therapy for HIV infection should be screened for evidence of hepatitis B and C and treated accordingly.
Drug Class: Antiviral Agents
Other Drugs in the Subclass, Integrase Strand Transfer Inhibitors: Cabotegravir, Dolutegravir, Elvitegravir, Raltegravir
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bictegravir – Biktarvy®
(Bictegravir, Emtricitabine and Tenofovir Alafenamide Combination Product)
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Bictegravir | 1611493-60-7 | C21-H18-F3-N3-O5 |
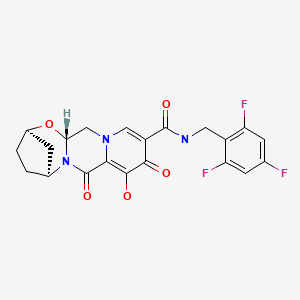
|
| Dolutegravir | 1051375-16-6 | C20-H19-F2-N3-O5 |
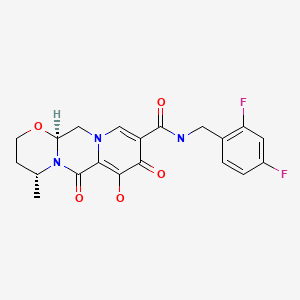
|
| Elvitegravir | 697761-98-1 | C23-H23-Cl-F-N-O5 |
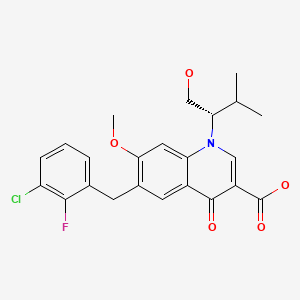
|
| Raltegravir | 518048-05-0 | C20-H21-F-N6-O5 |

|
ANNOTATED BIBLIOGRAPHY
References updated: 10 April 2019
- Núñez M. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 505-18.(Review of hepatotoxicity of antiviral agents including drugs for HIV infection, although the integrase strand transfer inhibitors are not discussed).
- Flexner C. Antiretroviral agents and treatment of HIV infection. In, Brunton LL, Hillal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1137-57.(Textbook of pharmacology and therapeutics).
- https://hivinfo
.nih.gov /hiv-source/medical-practice-guidelines /hiv-treatment-guidelines . (Regularly updated clinical guidelines on the use of antiretroviral agents in HIV-1 infected adults, adolescents and children). - Cocohoba J, Dong BJ. Raltegravir: the first HIV integrase inhibitor. Clin Ther. 2008;30:1747–65. [PubMed: 19014832](Review of the mechanism of action, pharmacokinetics and phase I-III studies of raltegravir; in combined phase III studies, ALT elevations above 5 times ULN occurred in 4.3% of 462 raltegravir treated vs 3.4% of 237 placebo treated patients who were receiving optimized background therapy; no hepatic serious adverse events were reported).
- Surgers L, Lacombe K. Hepatoxicity of new antiretrovirals: a systematic review. Clin Res Hepatol Gastroenterol. 2013;37:126–33. [PubMed: 23522569](Review of evidence of liver injury in large clinical trials of newer antiretroviral agents including dolutegravir but not bictegravir concluded that "the overall hepatic tolerance is far better with the novel drugs in this review than with former ARV regimens"; ALT elevations occurred with equal frequency in dolutegravir and comparator arms, and one patient on raltegravir developed DRESS syndrome).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 [1.3%] cases were attributed to antiretroviral agents, but none to the integrase inhibitors–dolutegravir, elvitegravir, raltegravir or bictegravir).
- Sax PE, Pozniak A, Montes ML, Koenig E, DeJesus E, Stellbrink HJ, Antinori A, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073–82. [PubMed: 28867499](Among 657 previously untreated adults with HIV infection who were started on combination antiretroviral therapy with bictegravir or dolutegravir with tenofovir and emtricitabine, HIV RNA suppression was similar in the two groups [89% vs 93%] as were the rates of adverse events, including ALT levels above 5 times ULN [2% vs 1%] which were usually attributable to concurrent hepatitis B or C).
- Gallant J, Lazzarin A, Mills A, Orkin C, Podzamczer D, Tebas P, Girard PM, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063–72. [PubMed: 28867497](Among 631 adults with previously untreated HIV infection started on single tablet regimens, viral control [HIV RNA <500 copies/mL] at 48 weeks was similar with bictegravir/emtricitabine/tenofovir [92%] as with dolutegravir/abacavir/lamivudine [93%], while adverse event rates were slightly less, including discontinuations for adverse events [0% vs 1.3%], serious adverse events [6% vs 8%] and ALT elevations above 5 times ULN [0.6% vs 1.3%]).
- Daar ES, DeJesus E, Ruane P, Crofoot G, Oguchi G, Creticos C, Rockstroh JK, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48-week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e347–e356. [PubMed: 29925490](Among 577 adults with HIV infection switched to Biktarvy [bictegravir/ emtricitabine/ tenofovir] or maintained on previous combination regimen, HIV RNA suppression was similar in the two groups, as were overall as well as serious adverse event rates, ALT elevations above 5 times ULN occurring in 1% vs 2%).
- Molina JM, Ward D, Brar I, Mills A, Stellbrink HJ, López-Cortés L, Ruane P, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48-week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e357–e365. [PubMed: 29925489](Among 563 adults with HIV infection switched to bictegravir/emtricitabine/tenofovir or maintained on previous combination regimen, HIV RNA suppression was similar in the two groups [both 99%], as were overall [80% vs 80%] as well as serious adverse event rates [5% vs 8%], ALT elevations above 5 times ULN occurring in 2% vs 0%, all abnormalities occurring in patients with underlying pre-existing liver disease).
- Markham A. Bictegravir: first global approval. Drugs. 2018;78:601–6. [PubMed: 29564777](Review of the mechanism of action, history of development, pharmacology, clinical efficacy and safety of bictegravir; mentions that elevations in serum ALT levels above 5 times ULN occurred in 1-2% of patients treated with bictegravir in clinical trials).
- Biktarvy--another INSTI-based combination for HIV. Med Lett Drugs Ther. 2018;60(1553):132–5. [PubMed: 30133424](Concise review of the mechanism of action, efficacy, safety and costs of bictegravir combined with tenofovir and emtricitabine [Biktarvy]; mentions the flares of hepatitis B in co-infected subjects can occur when the combination is stopped).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Dolutegravir.[LiverTox: Clinical and Researc...]Review Dolutegravir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- A New Mechanism of Resistance of Human Immunodeficiency Virus Type 2 to Integrase Inhibitors: A 5-Amino-Acid Insertion in the Integrase C-Terminal Domain.[Clin Infect Dis. 2019]A New Mechanism of Resistance of Human Immunodeficiency Virus Type 2 to Integrase Inhibitors: A 5-Amino-Acid Insertion in the Integrase C-Terminal Domain.Le Hingrat Q, Collin G, Lê M, Peytavin G, Visseaux B, Bertine M, Tubiana R, Karmochkine M, Valin N, Collin F, et al. Clin Infect Dis. 2019 Aug 1; 69(4):657-667.
- Comparison of the Antiviral Activity of Bictegravir against HIV-1 and HIV-2 Isolates and Integrase Inhibitor-Resistant HIV-2 Mutants.[Antimicrob Agents Chemother. 2...]Comparison of the Antiviral Activity of Bictegravir against HIV-1 and HIV-2 Isolates and Integrase Inhibitor-Resistant HIV-2 Mutants.Smith RA, Raugi DN, Wu VH, Zavala CG, Song J, Diallo KM, Seydi M, Gottlieb GS, University of Washington-Dakar HIV-2 Study Group. Antimicrob Agents Chemother. 2019 May; 63(5). Epub 2019 Apr 25.
- Antiviral Activity of Bictegravir and Cabotegravir against Integrase Inhibitor-Resistant SIVmac239 and HIV-1.[Antimicrob Agents Chemother. 2...]Antiviral Activity of Bictegravir and Cabotegravir against Integrase Inhibitor-Resistant SIVmac239 and HIV-1.Hassounah SA, Alikhani A, Oliveira M, Bharaj S, Ibanescu RI, Osman N, Xu HT, Brenner BG, Mesplède T, Wainberg MA. Antimicrob Agents Chemother. 2017 Dec; 61(12). Epub 2017 Nov 22.
- Review A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection.[Retrovirology. 2022]Review A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection.Zhao AV, Crutchley RD, Guduru RC, Ton K, Lam T, Min AC. Retrovirology. 2022 Oct 22; 19(1):22. Epub 2022 Oct 22.
- Bictegravir - LiverToxBictegravir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...