NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Betrixaban is an oral anticoagulant and direct inhibitor of factor Xa which is used to decrease the risk of deep vein thrombosis and pulmonary embolus in patients hospitalized with an acute medical condition who are at high risk for venous thromboses. Betrixaban has been linked to a low rate of serum aminotransferase elevations during therapy but has not been linked to instances of clinically apparent liver injury.
Background
Betrixaban (be trix' a ban) is an orally available, small molecule direct inhibitor of coagulation factor Xa (-xaban), the rate controlling last step in the generation of thrombin, the final intermediate in blood coagulation. Inhibiting thrombin prevents the conversion of fibrinogen to fibrin and subsequent cross linking of fibrin monomers, platelet activation and amplification of coagulation. Clinical trials have shown that anticoagulation with betrixaban can decrease the risk of deep vein thrombosis and pulmonary embolism in patients undergoing hospitalized with an acute medical illness that places them at high risk for thromboembolism. Betrixaban was approved for use in the United States in 2017 for prophylaxis of thromboembolism in patients hospitalized for an acute medical illness who are at moderate or high risk for thromboembolism. Betrixaban is available in 40 and 80 mg capsules under the commercial name Bevyxxa. The recommended dose is an initial single dose of 160 mg on day 1 followed by 80 mg once daily for 35 to 42 days. Lower doses are recommended for patients with renal dysfunction or on inhibitors of P-glycoprotein. Unlike warfarin, betrixaban does not require monitoring of bleeding time or INR. Side effects are not common, but can include bleeding, headache, dizziness, fatigue, gastrointestinal upset, nausea, arthralgias and rash. Uncommon, but potentially severe adverse events include severe bleeding episodes, epidural or spinal hematomas, and hypersensitivity reactions.
Hepatotoxicity
In registration studies, serum aminotransferase elevations of greater than 3 times the upper limit of normal (ULN) occurred in 1% to 2% of betrixaban-treated patients and in a similar proportion of control subjects treated with enoxaparin. Similarly, aminotransferase levels rose to above 5 times ULN in 0.6% of betrixaban and 0.4% of enoxaparin treated control subjects. Aminotransferase elevations with jaundice arose in <0.2% of patients in both groups and all cases appeared to be due to heart disease and congestive liver injury and unrelated to the anticoagulants used. Since approval and more widespread clinical use, there have been no reports of clinically apparent liver injury due to betrixaban. On the other hand, other direct factor Xa inhibitors, such as apixaban and rivaroxaban, have been linked to rare instances of idiosyncratic liver injury, generally arising within days or a few weeks of starting and presenting with a mild-to-moderate hepatocellular injury without prominent immunoallergic and autoimmune features. This syndrome has not been reported with betrixaban, but it has had limited clinical use.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The causes of the mild serum aminotransferase elevations during betrixaban therapy are unknown. Betrixaban has minimal hepatic metabolism and has no drug-drug interactions with substrates or modulators the cytochrome P450 system. Betrixaban is, however, a substrate of P-glycoprotein, potent inhibitors of which (such as amiodarone, azithromycin, clarithromycin, verapamil, ketoconazole) can cause elevated levels of betrixaban, increasing the risk of bleeding.
Outcome and Management
Betrixaban has been associated with mild, asymptomatic and self-limited elevations in serum aminotransferases but not with hepatitis with jaundice. There have been no reports of fulminant hepatic failure attributed to betrixaban or cases of chronic hepatitis or vanishing bile duct syndrome. In some instances, patients with acute liver injury due to one direct factor Xa inhibitor (rivaroxaban) have tolerated another (apixaban) without recurrence of liver abnormalities.
Drug Class: Antithrombotic Agents, Anticoagulants
Other Drugs in the Subclass, Anticoagulants, Factor Xa Antagonists: Apixaban, Edoxaban, Fondaparinux, Rivaroxaban
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Betrixaban – Generic, Bevyxxa®
DRUG CLASS
Antithrombotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
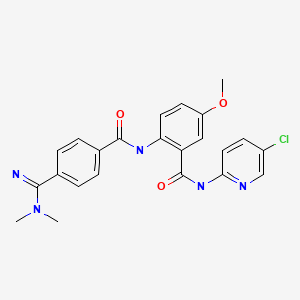
| Betrixaban | 330942-05-7 | C23-H22-Cl-N5-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 16 February 2023
- Zimmerman HJ. Platelet aggregation inhibitors. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 639-42.(Textbook of hepatotoxicity published in 1999 well before the availability of betrixaban and the direct Factor Xa inhibitors).
- De Marzio DH, Navarro VJ. Antiplatelet agents. Hepatotoxicity of cardiovascular and antidiabetic drugs: antihypertensives. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 527-8.(Review of hepatotoxicity of cardiovascular drugs does not discuss the anticoagulants).
- Hogg K, Weitz JI. Blood coagulation and anticoagulant, fibrinolytic, and antiplatelet drugs. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 585-604.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that rates of serum aminotransferase elevations during betrixaban therapy were similar to rates in patients treated with comparator anticoagulants [1.7% vs 1.5%] and the rare instances of clinically apparent liver injury with jaundice were all attributable to other causes such as heart failure and bacterial sepsis). - Turpie AG, Bauer KA, Davidson BL, Fisher WD, Gent M, Huo MH, Sinha U, et al. EXPERT Study Group. A randomized evaluation of betrixaban, an oral factor Xa inhibitor, for prevention of thromboembolic events after total knee replacement (EXPERT). Thromb Haemost. 2009;101:68–76. [PubMed: 19132191](Among 215 patients undergoing knee replacement surgery treated with betrixaban [15 or 40 mg twice daily] or enoxaparin [30 mg sc every 12 hours] for 10-14 days, venous thromboembolism was more frequent with betrixaban [15% and 20%] than enoxaparin [10%]; no mention of ALT elevations or hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were due to anticoagulants).
- Caldeira D, Barra M, Santos AT, de Abreu D, Pinto FJ, Ferreira JJ, Costa J. Risk of drug-induced liver injury with the new oral anticoagulants: systematic review and meta-analysis. Heart. 2014;100:550–6. [PubMed: 24476812](Systematic review of 29 controlled trials of oral anticoagulants in 152,116 patients focusing on risk of drug induced liver injury [including 7 trials with 22,992 patients on apixaban] found no increase in rate of serum ALT or AST elevations above 3 times ULN [1.0% vs 1.2%], or combined enzyme and bilirubin elevations above 2 times ULN [both 0.2%] with apixaban therapy compared to control patients on standard therapy).
- Liakoni E, Rätz Bravo AE, Krähenbühl S. Hepatotoxicity of new oral anticoagulants (NOACs). Drug Saf. 2015;38:711–20. [PubMed: 26138527](Systematic review of evidence of hepatotoxicity of new oral anticoagulants including rivaroxaban, apixaban, edoxaban and dabigatran found 22 cases of liver injury due to rivaroxaban, 2 dabigatran, 2 apixaban, but none to edoxaban).
- Anastasia EJ, Rosenstein RS, Bergsman JA, Parra D. Use of apixaban after development of suspected rivaroxaban-induced hepatic steatosis; a case report. Blood Coagul Fibrinolysis. 2015;26:699–702. [PubMed: 26154612](67 year old man developed ALT elevations 6 months after starting rivaroxaban [peak ALT 391 U/L, Alk P 120 U/L, bilirubin 1.3 mg/dL], which fell into the normal range within 2 months of switching to apixaban; ultrasound suggested fatty liver which also resolved with stopping).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, two cases were attributed to anticoagulants(prasugrel and dalteparin), but none to direct factor Xa antagonists).
- Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, Gold A, Hernandez AF, Gibson CM. APEX Investigators. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534–44. [PubMed: 27232649](Among 7513 patients hospitalized with acute medical illness and at risk of venous thromboembolism treated with betrixaban [80 mg daily for 35-42 days] or enoxaparin [40 mg sc once daily for 10-14 days], evidence of venous thromboembolism was found in 69% on betrixaban and 8.5% on enoxaparin; no mention of ALT elevations or hepatotoxicity).
- Garland SG, DeRemer CE, Smith SM, Gums JG. Betrixaban: a new oral factor Xa inhibitor for extended venous thromboembolism prophylaxis in high-risk hospitalized patients. Ann Pharmacother. 2018;52:554–61. [PubMed: 29338293](Review of the pharmacology, clinical efficacy and safety of betrixaban; no mention of ALT elevations or hepatoxicity).
- Comparison table: some oral anticoagulants for VTE. Med Lett Drugs Ther. 2018;60(1542):e51–e54. [PubMed: 29537394](Table of oral anticoagulants used for VTE compares, indications, doses, adverse events and special issues; mentions adverse events of aminotransferase elevations for apixaban, edoxaban, rivaroxaban but not betrixaban or dabagatran).
- Betrixaban (Bevyxxa) for VTE prophylaxis in acute medical illness. Med Lett Drugs Ther. 2018;60(1537):4–5. [PubMed: 29294463](Concise review of the currently approved drugs for prevention of venous thromboembolism and the clinical efficacy, safety and costs of betrixaban; no mention of ALT elevations or hepatotoxicity).
- Drugs for treatment and prevention of venous thromboembolism. Med Lett Drugs Ther. 2018;60:41–8. [PubMed: 29537392](Concise review of drugs approved for venous thromboembolism; mentions that betrixaban is approved for prophylaxis only and not treatment; no mention of hepatic side effects).
- Björnsson HK, Gudmundsson DO, Björnsson ES. Liver injury caused by oral anticoagulants: A population-based retrospective cohort study. Liver Int. 2020;40:1895–1900. [PubMed: 32511827](Search of Icelandic National Prescription Databases and Health Records identified 3 cases of clinically apparent, acute liver injury in 3 of 3446 [0.1%] patients taking rivaroxaban, but in none of 9101 taking warfarin, 1903 apixaban, 1335 dabigatran or 34 taking edoxaban; betrixaban was not mentioned).
- Yang X, Li N, Guo T, Guan X, Tan J, Gao X, Wu Y, Jia L, et al. Comparison of the effects of low-molecular-weight heparin and fondaparinux on liver function in patients with pulmonary embolism. J Clin Pharmacol. 2020;60:1671–1678. [PubMed: 32639644](Among 463 adults with pulmonary embolus treated with low molecular weight heparins or with fondaparinux, 79 [17%] developed evidence of liver injury that was generally mild and less than 3 times ULN in 98%, including 15% of 377 on edoxaban, 32% of 59 on nadroparin, and 7.4% of 27 on fondaparinux; no mention of betrixaban).
- Zhao J, Blais JE, Chui CSL, Suh IH, Chen EYH, Seto WK, Mok MT, et al. Association between nonvitamin K antagonist oral anticoagulants or warfarin and liver injury: a cohort study. Am J Gastroenterol. 2020;115:1513–1524. [PubMed: 32467502](Among 13,698 patients with atrial fibrillation started on anticoagulant therapy and followed in the Hong Kong Clinical Database and Reporting system between 2010 and 2016, 513 [2.8%] developed evidence of liver injury and propensity matching indicated that liver injury was less frequent [2.1% vs 3.4%], although more severe with oral anticoagulants than with warfarin).
- Maura G, Bardou M, Billionnet C, Weill A, Drouin J, Neumann A. Oral anticoagulants and risk of acute liver injury in patients with nonvalvular atrial fibrillation: a propensity-weighted nationwide cohort study. Sci Rep. 2020;10:11624. [PMC free article: PMC7363898] [PubMed: 32669591](Among 434,015 patients with atrial fibrillation started on anticoagulant therapy and followed in the French National Healthcare Database, 218 [0.6%] were subsequently hospitalized for acute liver injury, the rates being similar for those on dabigatran [26 of 51,737 patients], apixaban [29 of 62,503 patients] and rivaroxaban [46 of 99,408 patients]; no mention of betrixaban).
- Zhou J, Leung KSK, Kong D, Lee S, Liu T, Wai AKC, Chang C, et al. Low rates of liver injury in edoxaban users: Evidence from a territory-wide observational cohort study. Clin Cardiol. 2021;44:886–889. [PMC free article: PMC8259145] [PubMed: 33590891](Among 1213 patients with atrial fibrillation started on edoxaban between 2016 and 2020 and followed in the Hong Kong Clinical Database and Reporting System, 19 [1.5%] developed evidence of liver injury, a rate below that previously reported for warfarin [3.7%], apixaban [2.5%], and rivaroxaban [2.1%]).
- Ma J, Chalasani NP, Schwantes-An L, Björnsson ES. Review article: the safety of anticoagulants and antiplatelet agents in patients with cirrhosis. Aliment Pharmacol Ther. 2023;57:52–71. [PubMed: 36373544](Review of the safety of anticoagulant use in patients with cirrhosis mentions that drug induced liver injury is uncommon in patients with preexisting cirrhosis, at least in those with compensated [Child’s Class A] or mildly compensated [Child’s Class B] cirrhosis).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Apixaban.[LiverTox: Clinical and Researc...]Review Apixaban.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Edoxaban.[LiverTox: Clinical and Researc...]Review Edoxaban.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Betrixaban: A New Oral Factor Xa Inhibitor for Extended Venous Thromboembolism Prophylaxis in High-Risk Hospitalized Patients.[Ann Pharmacother. 2018]Betrixaban: A New Oral Factor Xa Inhibitor for Extended Venous Thromboembolism Prophylaxis in High-Risk Hospitalized Patients.Garland SG, DeRemer CE, Smith SM, Gums JG. Ann Pharmacother. 2018 Jun; 52(6):554-561. Epub 2018 Jan 17.
- Oral direct factor Xa inhibitor versus enoxaparin for thromboprophylaxis after hip or knee arthroplasty: Systemic review, traditional meta-analysis, dose-response meta-analysis and network meta-analysis.[Thromb Res. 2015]Oral direct factor Xa inhibitor versus enoxaparin for thromboprophylaxis after hip or knee arthroplasty: Systemic review, traditional meta-analysis, dose-response meta-analysis and network meta-analysis.Feng W, Wu K, Liu Z, Kong G, Deng Z, Chen S, Wu Y, Chen M, Liu S, Wang H. Thromb Res. 2015 Dec; 136(6):1133-44. Epub 2015 Oct 20.
- Review Betrixaban: a direct oral inhibitor of activated factor X for the prophylaxis of venous thromboembolism in patients hospitalized for acute medical illness.[Drugs Today (Barc). 2017]Review Betrixaban: a direct oral inhibitor of activated factor X for the prophylaxis of venous thromboembolism in patients hospitalized for acute medical illness.Escolar G, Díaz-Ricart M, Arellano-Rodrigo E. Drugs Today (Barc). 2017 Aug; 53(8):423-434.
- Betrixaban - LiverToxBetrixaban - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...