NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Apomorphine is a subcutaneously administered dopamine receptor agonist used predominantly in the therapy of hypomobility of advanced Parkinson disease. The use of apomorphine has been limited, but it has not been associated with serum enzyme elevations during treatment nor has it been implicated in cases of acute liver injury.
Background
Apomorphine (a" poe mor' feen) is a subcutaneously administered dopamine receptor agonist which has moderate affinity for the D2, D3, and D5 class of dopamine receptors in the central nervous system and little activity against the D1 class. It also has some alpha adrenergic activity. Apomorphine was shown to improve motor function in animal models of Parkinson disease and in clinical trials, was shown to decrease hypomobility in patients with advanced Parkinsonism. Apomorphine was approved for use in the United States in 2004, but had been used in Europe for more than a decade. Current indications are for acute and intermittent treatment of hypomobility of advanced Parkinson disease. It is also used for acute dystonic reactions. Apomorphine is available in a liquid solution of 10 mg/mL under the brand name Apokyn. It is given in 0.2 to 0.6 mL doses subcutaneously as needed up to 3 times daily. Apomorphine injections usually cause nausea and vomiting requiring antiemetics. It can cause hypotension, gastrointestinal upset, anxiety, confusion, dizziness, headache, hallucinations, vivid dreams and insomnia, symptoms typical of dopaminergic stimulation.
Hepatotoxicity
Apomorphine has not been reported to cause serum aminotransferase elevations or clinically apparent acute liver injury, but its use has been limited and is typically given in low doses for a limited period of time. Thus, if apomorphine causes liver injury it must be rare.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The metabolism of apomorphine has not been well defined; it appears to be minimally metabolized in the liver.
Drug Class: Antiparkinson Agents
Other Drugs in the Subclass, Dopamine Receptor Agonists: Bromocriptine, Pergolide, Pramipexole, Ropinirole, Rotigotine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Apomorphine – Apokyn®
DRUG CLASS
Antiparkinson Agents
Product labeling at DailyMed, National Library of Medicine, NIH
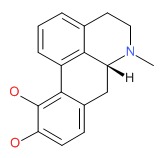
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Apomorphine | 58-00-4 | C17-H17-N-O2 |
 |
REFERENCES
References updated: 20 July 2017
- Zimmerman HJ. Antiparkinsonism drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 715-7.(Expert review of hepatotoxicity published in 1999; among anticholinergic agents, "only trihexyphenidyl has been incriminated in hepatic injury"; other antiparkinsonism drugs discussed include levodopa, lergotrile [no longer available], pergolide and bromocriptine).
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier Inc, 2013, pp. 443-62.(Review of hepatotoxicity of agents acting on the central nervous system).
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 609-28.(Textbook of pharmacology and therapeutics).
- McDowell F. Symposium on levodopa in Parkinson's disease. Clinical and pharmacological aspects. Clinical laboratory abnormalities. Clin Pharmacol Ther 1971; 12: 335-9. [PubMed: 4102803](Retrospective analysis of laboratory abnormalities arising in 974 patients with Parkinson disease treated with levodopa; AST elevations occurred in 9% of 5427 determinations, but were usually mild and transient returning to normal in 1-2 months without dose adjustment; AST levels rose to 1600 U/L in one patient who later died of complications of diabetes).
- Pinter MM, Helscher RJ, Mundsperger N, Binder H. Transient increase of pancreatic enzymes evoked by apomorphine in Parkinson's disease. J Neural Transm 1998; 105: 1237-44. [PubMed: 9928892](Among 29 patients with Parkinson disease treated with apomorphine, transient asymptomatic elevations in amylase and lipase elevations occurred in 5; liver enzymes remained normal).
- Lambert D, Waters CH. Comparative tolerability of the newer generation antiparkinsonian agents. Drugs Aging 2000; 16: 55-65. [PubMed: 10733264](Review of mechanism of action, tolerability and safety of selegiline, pramipexole, ropinirole, tolcapone and entacapone in Parkinson disease).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to agents used for Parkinson disease).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25,1425. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96 were attributed to an agent used to treat Parkinson disease).
- Drugs for Parkinson's disease. Treat Guidel Med Lett 2013; 11 (135): 101-6. [PubMed: 24165688](Concise review of recommendations for therapy of Parkinson disease with description of mechanisms of action, efficacy and adverse events).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to an agent to treat Parkinson disease).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury from the US enrolled in a prospective database between 2004 and 2012, none were attributed to an agent used to treat Parkinson disease).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Update on apomorphine for the rapid treatment of hypomobility ("off") episodes in Parkinson's disease.[Pharmacotherapy. 2006]Review Update on apomorphine for the rapid treatment of hypomobility ("off") episodes in Parkinson's disease.Obering CD, Chen JJ, Swope DM. Pharmacotherapy. 2006 Jun; 26(6):840-52.
- Review [Apomorphine in the treatment of Parkinson's Disease].[Nervenarzt. 2005]Review [Apomorphine in the treatment of Parkinson's Disease].Dressler D. Nervenarzt. 2005 Jun; 76(6):681-9.
- Review Use of apomorphine in Parkinson's disease.[Hosp Med. 1999]Review Use of apomorphine in Parkinson's disease.O'Sullivan JD, Lees AJ. Hosp Med. 1999 Nov; 60(11):816-20.
- Review Subcutaneous apomorphine : an evidence-based review of its use in Parkinson's disease.[Drugs Aging. 2004]Review Subcutaneous apomorphine : an evidence-based review of its use in Parkinson's disease.Deleu D, Hanssens Y, Northway MG. Drugs Aging. 2004; 21(11):687-709.
- [Apomorphine in the treatment of Parkinson disease].[Ugeskr Laeger. 1991][Apomorphine in the treatment of Parkinson disease].Christensen PB, Dupont E, Jensen NB. Ugeskr Laeger. 1991 Sep 16; 153(38):2631-4.
- Apomorphine - LiverToxApomorphine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...