NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Allopurinol is a xanthine oxidase inhibitor and a widely used medication for gout. Allopurinol is a rare but well known cause of acute liver injury that has features of a hypersensitivity reaction and can be severe and even fatal.
Background
Allopurinol (al' oh pure' i nol) is an analog of hypoxanthine and a potent inhibitor of the enzyme xanthine oxidase that is responsible for converting hypoxanthine to xanthine and xanthine to uric acid in the breakdown pathway of purines. Allopurinol lowers serum and tissue uric acid levels and has potent activity against gout, largely in preventing rather than treating acute attacks of gout. Allopurinol was approved for use in the United States in 1963 and is still widely used. Current indications include therapy and prevention of symptomatic gout, uric acid nephropathy, and the hyperuricemia caused by malignancy and anticancer therapy. It is not recommended for treatment of asymptomatic hyperuricemia. Allopurinol is available in multiple generic forms and under the brand name of Zyloprim or Aloprim in tablets of 100 and 300 mg. Intravenous formulations are also available. The recommended initial dose for therapy of gout is 100 mg daily, with increases of 100 mg in daily dose weekly until uric acid levels fall to 6 mg/dL or below, but not to exceed 800 mg daily. The average daily dose in therapy of gout is 300 mg. Common side effects include skin rash and hypersensitivity reactions. Rare but potentially severe adverse events include severe cutaneous reactions including drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN).
Hepatotoxicity
Chronic therapy with allopurinol is associated with transient and minor liver test abnormalities in 2% to 6% of patients, which resolve spontaneously or with drug discontinuation. More importantly, allopurinol has been linked to a very distinctive form of acute liver injury that is accompanied by prominent immunoallergic manifestations such as fever, rash, eosinophilia, lymphadenopathy, atypical lymphocytosis, thrombocytopenia, arthralgias and facial edema (drug reaction with eosinophilia and systemic symptoms — DRESS syndrome) (Case 1). The typical latency to onset is 2 to 8 weeks and liver injury arising during long term therapy is uncommon. The pattern of liver enzyme elevations tends to be mixed, but can be hepatocellular or purely cholestatic. Autoantibodies are not common. In some cases rash and fever arise before evidence of liver injury and rises in serum enzymes and bilirubin occur 1 to 2 weeks after the first immunoallergic manifestations. Eosinophilia in addition may arise only after the clinical manifestations. The systemic symptoms and signs of DRESS syndrome caused by allopurinol can also be manifested by renal, pulmonary or pancreatic dysfunction and even acalculous cholecystitis. More severe forms of allopurinol hypersensitivity reactions include Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), both of which are commonly accompanied by signs of liver injury, although the liver injury is often mild and transient serum aminotransferase elevations without jaundice. Overall, allopurinol hypersensitivity reactions have a high fatality rate, either from acute liver failure, chronic cholestatic injury or complications of other allergic manifestations such as toxic epidermal necrolysis, vasculitis, pancreatitis and renal dysfunction. African-American race and preexisting renal disease appear to be risk factors for hypersensitivity reactions to allopurinol.
Likelihood score: A (well established cause of clinically apparent liver injury).
Histopathology
Liver biopsy in allopurinol hepatotoxicity typically shows an acute cholestatic or mixed hepatitis. Bile duct injury may be prominent early and loss of bile ducts later during the course. Histology can also show granulomas including "ring" granulomas that are typically associated with visceral infections such as Q fever or Kala-azar. Granulomas may be found in other organs as well and represent a typical histological correlate to the immunoallergic response to a medication. Two examples of allopurinol hepatotoxicity are shown: one with a cholestatic hepatitis and another with acute granulomatous changes.
CHOLESTATIC HEPATITIS

| Allopurinol may cause cholestatic hepatitis. This case shows canalicular (arrow) and hepatocellular cholestasis in zone 3. Only very mild inflammation is present in this photo. The central vein (V) is indicated. |
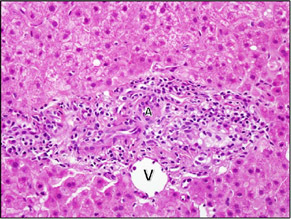
| In this case, there was mild portal inflammation, mainly composed of lymphocytes. In over half the portal areas, no duct could be found, consistent with a vanishing bile duct syndrome. This portal area only shows an artery (A) and vein (V). |
GRANULOMATOUS HEPATITIS

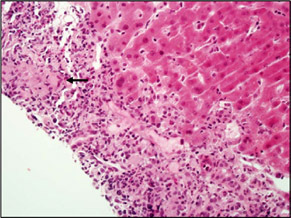
| This case had granulomas in almost all of the portal areas. In this portal, the epithelioid macrophages (arrow) of the granulomas are in the center part of the portal area. The granuloma is surrounded by a mixed inflammatory infiltrate of lymphocytes, neutrophils and eosinophils. A cluster of eosinophils is indicated by the arrowhead. |
| Another portal area showing a granuloma (arrow) along with mixed inflammation. |
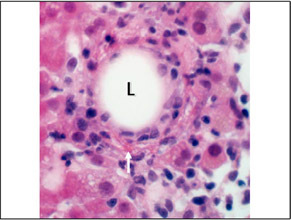
| A fibrin‐ring granuloma was present in this case. A fibrin‐ring granuloma is a granuloma that forms around a lipid droplet (L). A thin, irregular, ring of brightly eosinophilic fibrin can be seen running circumferentially around the lipid drop. It is best seen at the bottom (arrow). |
Mechanism of Injury
The mechanism of allopurinol hepatotoxicity is believed to be immunoallergic. Many cases resemble those of anticonvulsant hypersensitivity. Recently, several cases have been linked to concurrent infection with human herpesvirus-6, EBV or CMV infections. Severe allopurinol hypersensitivity skin reactions have been closely linked to HLA B*58:01 particularly in Asian populations but also to a lesser extend in Caucasians and African Americans, in whom there are also associations HLA-B*53:01 and A*34.02. These HLA associations are less common in cases of allopurinol liver injury without DRESS syndrome or SJS/TEN.
Outcome and Management
While most cases of acute liver injury attributed to allopurinol are self-limited and start to resolve within 7 to 10 days of stopping the medication, other cases are protracted, severe and even fatal. Instances of chronic vanishing bile duct syndrome due to allopurinol have been reported. Because of the accompanying allergic manifestations, corticosteroids are often used and usually result in prompt improvements in fever and rash, but their efficacy in ameliorating the liver injury is unproven. Relapse with early discontinuation of corticosteroids is common. There is no known cross reactivity of hypersensitivity to allopurinol with similar reactions to other medications, including the anticonvulsants.
Drug Class: Antigout Agents
CASE REPORTS
Case 1. DRESS syndrome accompanied by acute liver failure due to allopurinol.(1)
A 58 year old woman with diabetes, hypertension, gastric ulcer and gouty arthritis with hyperuricemia and mild renal insufficiency was started on allopurinol (300 mg daily) and developed fever and rash 17 days later. She had undergone resection of a parathyroid adenoma under enflurane anesthesia shortly after starting allopurinol, but she recovered uneventfully and was sent home on doxycycline, in addition to her usual medications including glibenclamide, indomethacin and cimetidine. One week later, she developed fever, fatigue and rash which became generalized and exfoliative. Allopurinol was stopped and she was admitted for observation. She was markedly febrile (39oC) and had a generalized erythematous rash. Blood testing showed leukocytosis and eosinophilia. Liver tests, which were previously normal, were mildly elevated on admission, but over the next few days worsened with onset of jaundice (Table). Tests for hepatitis A and B were negative. She subsequently developed progressive prolongation of the prothrombin time followed by confusion, encephalopathy and ascites. Corticosteroids were started. She developed gram negative sepsis followed by multiorgan failure and died. Postmortem liver biopsy showed marked centrilobular necrosis, cholestasis, inflammation and small islands of regenerating hepatocytes.
Key Points
| Medication: | Allopurinol (300 mg daily) |
|---|---|
| Pattern: | Mixed (R=2.2) |
| Severity: | 5+ (death from hepatic failure) |
| Latency: | 3 weeks |
| Recovery: | None |
| Other medications: | Glibenclamide, indomethacin, and cimetidine chronically. Enflurane 2 weeks before onset, doxycycline for the 6 days before onset. |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Allopurinol started (300 mg daily) for gout | |||||
| 4 days | 25 | 100 | 0.5 | Preoperative testing | |
| 17 days | 0 | 110 | 120 | 0.5 | Admission: rash and fever |
| 4 weeks | 11 days | 1240 | 1240 | 12.0 | Prothrombin time 9 sec prolonged |
| 6 weeks | 25 days | 65 | 390 | 30.0 | Ascites, coma and sepsis |
| Normal Values | <40 | <95 | <1.2 | ||
Comment
This patient developed typical allopurinol hypersensitivity syndrome 3 weeks after starting therapy. Risk factors included preexisting renal insufficiency. Several days after being admitted for rash and fever, she developed jaundice and had subsequent worsening with development of hepatic failure. This syndrome is also referred to as DRESS (drug rash with eosinophilia and systemic symptoms) and is usually rapidly reversible with stopping the medication. However, the hypersensitivity reaction can be severe and result in death from acute liver failure or from complications of generalized skin rash (toxic epidermal necrolysis) or renal disease. Corticosteroid therapy is often given and usually results in rapid disappearance of fever and improvement in rash, but relapse with stopping corticosteroids is common and this therapy is of unproven benefit for the hepatic injury and can complicate management of acute liver failure. Severe cutaneous reactions to allopurinol have been linked to HLA B*58:01 and cases of hepatotoxicity with allergic manifestations and skin rash are likely to have a similar linkage.
Case 2. DRESS syndrome and mixed hepatitis due to allopurinol.(2)
A 61 year old African American woman with diabetes, hypertension, chronic congestive heart failure, obesity, chronic renal dysfunction, and asthma was started on gradually increasing doses of allopurinol for gout. Approximately 11 weeks later, she developed fatigue, followed by rash, fever and facial swelling. She had no history of liver disease or risk factors for viral hepatitis and did not drink alcohol. Her other medications included metformin, amlodipine, metoprolol, clonidine, furosemide, iron, fluticasone with salmeterol, warfarin and aspirin, all of which she had been taking for several years. On examination, she had fever to 39.2 oC and a diffuse maculopapular rash. Blood testing showed leukocytosis and eosinophilia and worsening of her chronic renal dysfunction. A skin biopsy showed vacuolar interface dermatitis and eosinophils consistent with a drug reaction. Initially, liver tests were normal or only minimally elevated with bilirubin 0.3 mg/dL, ALT 89 U/L and Alk P 102. Despite stopping allopurinol promptly, however, her liver tests worsened over the ensuing week, with bilirubin rising to 7.7 mg/dL, ALT to 414 U/L and Alk P to 243 U/L. Tests for hepatitis A, B, C and E, Epstein Barr virus and cytomegalovirus infection were negative as were ANA, SMA and AMA. Abdominal ultrasound and magnetic resonance imaging showed no evidence of gallstones or biliary dilation. She was treated with prednisone and her fever and rash improved but bilirubin worsened. A liver biopsy showed bland cholestasis and minimal inflammation, no duct loss and no fat or fibrosis. Serum bilirubin rose to a peak of 23.7 mg/dL and INR to 1.7 and she developed worsening renal function and suspected pneumonia. Serum bilirubin levels remained above 16 mg/dL and she developed pneumonia followed by progressive multiorgan failure and died 8 weeks after initial presentation.
Key Points
| Medication: | Allopurinol (100 to 800 mg daily) |
|---|---|
| Pattern: | Mixed (R=3.9) |
| Severity: | 5+ (death from multiorgan failure) |
| Latency: | 10 weeks |
| Recovery: | None |
| Other medications: | Metformin, amlodipine, metoprolol, clonidine, furosemide warfarin, aspirin, iron, fluticasone with salmeterol |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Allopurinol started (100 to 800 mg daily) for gout | |||||
| 11 weeks | 0 | 89 | 102 | 0.3 | Rash and fever |
| 3 months | 9 days | 331 | 220 | 0.5 | |
| 13 days | 430 | 234 | 5.6 | ||
| 16 days | 414 | 243 | 7.7 | US: normal | |
| 23 days | 273 | 289 | 10.7 | MRI: normal | |
| 33 days | 158 | 512 | 16.1 | INR 1.6 | |
| 4 months | 37 days | 132 | 438 | 16.8 | INR 1.2 |
| 41 days | 146 | 482 | 23.7 | ||
| 48 days | 165 | 262 | 16.1 | ||
| 55 days | 172 | 295 | 19.6 | ||
| 5 months | 56 days | Died: multiorgan failure | |||
| Normal Values | <45 | <35 | <1.2 | ||
Comment
This patient developed DRESS syndrome 11 weeks after starting allopurinol therapy of gradually increasing doses. Risk factors included preexisting renal insufficiency and African American race. Several days after being admitted for rash and fever, she developed worsening jaundice which did not respond to withdrawal of allopurinol or systemic corticosteroid therapy. Her persistent jaundice and downhill course appeared to be exacerbated by renal insufficiency and infectious and metabolic complications of high dose corticosteroids. Her death from multiorgan failure was not directly related to the liver injury, but the hepatic dysfunction definitely contributed to the poor outcome. Typical of this case was the onset of jaundice a week or two after onset of the rash and fever. Atypical was the somewhat long latency, the usual latency for allopurinol hypersensitivity reactions being 3 to 4 weeks. Testing of stored blood demonstrated that she was heterozygous for HLA-B*58:01, a known risk factor for allopurinol induced severe cutaneous reactions (both DRESS and Stevens-Johnson syndrome). The HLA type is relatively common among Chinese subjects (allele frequency [AF]: 0.09), less common among African Americans (AF: 0.04) and rare in European Americans (AF: <0.01), perhaps accounting for the racial differential in susceptibility to allopurinol hypersensitivity reactions.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Allopurinol – Generic, Aloprim®, Zyloprim®
DRUG CLASS
Antigout Agents/Gout Suppressants
Product labeling at DailyMed, National Library of Medicine, NIH
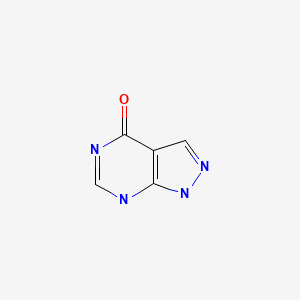
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Allopurinol | 315-30-0 | C5-H4-N4-O |
|
CITED REFERENCES
- 1.
- Raper R, Ibels L, Lauer C, Barnes P, Lunzer M. Fulminant hepatic failure due to allopurinol. Aust N Z J Med. 1984;14:63–5. [PubMed: 6590011]
- 2.
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159]
ANNOTATED BIBLIOGRAPHY
References updated: 26 December 2020
Abbreviations: DRESS, drug reaction with eosinophilia and systemic symptoms; SJS, Stevens Johnson Syndrome; TEN, toxic epidermal necrolysis.
- Zimmerman HJ. Drugs used to treat gout. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 543-4.(Expert review of allopurinol hepatotoxicity published in 1999; mentions that allopurinol has been implicated in 25 cases of liver injury, usually with fever, rash and eosinophilia arsing 3-6 weeks after starting, sometimes with granulomas on liver biopsy).
- Grosser T, Smyth E, FitzGerald GA. Pharmacology of gout. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 994-1004.(Textbook of pharmacology and therapeutics).
- Hall AP, Holloway VP, Scott JT. 4-hydroxypyrazolo (3,4-d) pyrimidine (HPP) in the treatment of gout: preliminary observations. Ann Rheum Dis. 1964;23:439–46. [PMC free article: PMC1030959] [PubMed: 14229577](Initial description of allopurinol effects in 4 patients with gout; one developed rash ~6 weeks after starting, resolving on stopping, but not recurring on rechallenge).
- Lidsky MD, Sharp JT. Jaundice with the use of 4-hydroxypyrazolo (3,4-d) pyrimidine. Arthritis Rheum. 1967;10:294.(Abstract: Among 14 patients with gout given allopurinol, 2 developed cholestatic hepatitis and several more had serum enzyme elevations; preexisting renal disease appeared to be a predisposing factor).
- Jarzobski J, Ferry J, Wombolt D, Fitch DM, Egan JD. Vasculitis with allopurinol therapy. Am Heart J. 1970;79:116–21. [PubMed: 5410273](76 year old man developed fever and severe exfoliative rash 4 weeks after starting allopurinol with 9% eosinophils, but normal AST and bilirubin, subsequently developed renal failure and died; autopsy showed hypersensitivity vasculitis involving kidneys, liver, spleen and lungs).
- Kantor GL. Toxic epidermal necrolysis, azotemia, and death after allopurinol therapy. JAMA. 1970;212:478–9. [PubMed: 5467301](72 year old man developed fever and severe rash 2 weeks after starting allopurinol [bilirubin and Alk P not given, ALT 65 U/L, 13% eosinophils, creatinine 3.6 mg/dL], treated with corticosteroids, but died of toxic epidermal necrolysis and sepsis; no mention of liver pathology).
- Mills RM. Severe hypersensitivity reactions associated with allopurinol. JAMA. 1971;216:799–802. [PubMed: 4252397](52 year old man and 59 year old woman developed fever and rash with eosinophilia and renal insufficiency 3 and 5 weeks after starting allopurinol, little documentation of liver involvement, both recovered but required prolonged corticosteroid therapy).
- Simmons F, Feldman B, Gerety D. Granulomatous hepatitis in a patient receiving allopurinol. Gastroenterology. 1972;62:101–4. [PubMed: 5059424](50 year old man developed fever with eosinophilia and enzyme elevations [ALT 320 U/L, Alk P 240 U/L] without jaundice 3 weeks after starting allopurinol; liver biopsy showed granulomas and focal necrosis, rapid resolution with stopping and no granulomas on two follow up biopsies 1 and 4 months later).
- Young JL, Boswell RB, Nies AS. Severe allopurinol hypersensitivity. Association with thiazides and prior renal compromise. Arch Intern Med. 1974;134:553–8. [PubMed: 4546912](40 year old man and 67 year old woman developed fever, rash and eosinophilia 4 weeks after starting allopurinol [bilirubin 2.0 and 1.8 mg/dL, AST 1550 and 210 U/L], one patient dying of liver [bilirubin rising to 15.0 mg/dL] and renal failure, and the other surviving; preexisting renal insufficiency, thiazide use and African-American race were thought to be risk factors).
- McMenamin RA, Davies LM, Craswell PW. Drug induced interstitial nephritis, hepatitis and exfoliative dermatitis. Aust N Z J Med. 1976;6:583–7. [PubMed: 139882](Among 4 cases of rash, fever, nephritis and hepatitis, one was linked to allopurinol, arising after 6 weeks of therapy [bilirubin 2.5 mg/dL, AST 215 U/L, Alk P 323 U/L, eosinophils 7%], with transient renal failure [creatinine 15 mg/dL], resolving after therapy with prednisone).
- Boyer TD, Sun N, Reynolds TB. Allopurinol-hypersensitivity vasculitis and liver damage. West J Med. 1977;126:143–7. [PMC free article: PMC1237483] [PubMed: 139760](29, 59 and 67 year old men developed rash and fever 4 weeks after starting allopurinol [bilirubin 14.4, 1.8 and 3.1 mg/dL, ALT 940, 165 and 150 U/L, Alk P 1-3x ULN], 2 had renal insufficiency, 1 case was fatal, and others recovered with prednisone treatment in 1-3 months; rechallenge with a single dose induced fever and rash without liver abnormalities).
- Espiritu CR, Alalu J, Glueckauf LG, Lubin J. Allopurinol-induced granulomatous hepatitis. Am J Dig Dis. 1976;21:804–6. [PubMed: 961676](55 year old woman developed fever and arthralgias [but no rash or eosinophilia] 4 weeks after starting allopurinol [bilirubin 0.9 mg/dL, ALT 140 U/L and Alk P 900 U/L] and biopsy showing granulomas; improved with stopping drug and had normal values and liver histology 3 months later during cholecystectomy).
- Chawla SK, Patel HD, Parrino GR, Soterakis J, Lopresti PA, D'Angelo WA. Allopurinol hepatotoxicity. Case report and literature review. Arthritis Rheum. 1977;20:1546–9. [PubMed: 921828](44 year old man developed abdominal pain a week after starting allopurinol [bilirubin 1.8 mg/dL, AST 50 U/L, Alk P 98 U/L, 8% eosinophils]; biopsy showed noncaseating granulomas; resolved rapidly with stopping therapy).
- Butler RC, Shah SM, Grunow WA, Tester EC. Massive hepatic necrosis in a patient receiving allopurinol. JAMA. 1977;237:473–4. [PubMed: 576272](48 year old woman developed urticaria, fever and rash 1-2 weeks after starting allopurinol [bilirubin 4.6 rising to 13.1 mg/dL, AST 880 U/L, Alk P 188 U/L], and progressive liver failure; autopsy showed massive hepatic necrosis).
- Shah SM, Butler RC, Grunow WA, Texter EC Jr. Massive hepatic necrosis in a patient receiving concomitant medication. JAMA. 1977;237:2036. [PubMed: 576881](Follow up of Butler [1977] listing concomitant medications, none of which were implicated because none had been recently changed).
- Swank LA, Chejfec G, Nemchausky BA. Allopurinol-induced granulomatous hepatitis with cholangitis and a sarcoid-like reaction. Arch Intern Med. 1978;138:997–8. [PubMed: 646570](36 year old man developed fever and polyarthralgias 1 month after starting allopurinol with leukocytosis [bilirubin 4.7 mg/dL, AST 145 U/L, Alk P 875 U/L] and liver biopsy showing multiple noncaseating granulomas and cholangitis, resolved on stopping and follow up liver biopsy was normal).
- Male PJ, Schaer B, Posternak R. Reaction d'hypersensibilite a l'allopurinol. Schweiz Med Wochenschr. 1978;108:661–3. [PubMed: 653331](71 year old man developed fever, rash, eosinophilia and lymphadenopathy 20 days after starting allopurinol [bilirubin 25 mg/dL, ALT "without perturbation", Alk P 252 U/L, abnormal renal function], who was treated with prednisone and recovered).
- Medline A, Cohen LB, Tobe BA, Sellers EM. Liver granulomas and allopurinol. Br Med J. 1978;1:1320–1. [PMC free article: PMC1604728] [PubMed: 647256](47 year old man on allopurinol for 6 years developed fever and abdominal pain [bilirubin 1.4 mg/dL, ALT 53 U/L, Alk P 3 times ULN, eosinophilia of 7%, ESR 80], who upon cholecystectomy had no stones, but liver biopsy showed multiple noncaseating granulomas and giant cells, resolved on stopping allopurinol and follow up biopsy showed no granulomas).
- Korting HC, Lesch R. Acute cholangitis after allopurinol treatment. Lancet. 1978;1:275–6. [PubMed: 74697](48 year old man with renal insufficiency developed rash, fever and abdominal pain 4-5 weeks after starting allopurinol [bilirubin 1.1 rising to 7.7 mg/dL, ALT 134 U/L, Alk P 1317 U/L, 10% eosinophilia], liver biopsy showing cholangitis and cholestasis, died of multiorgan failure).
- Haughey DB, Lanse S, Imhoff T, Tobin M, Schentag JJ. Allopurinol sensitivity: report of two cases. Am J Hosp Pharm. 1979;36:1377–80. [PubMed: 159623](2 men, ages 72 and 58 years, developed fever, rash and eosinophilia 2 and 4 weeks after starting allopurinol [bilirubin 2.4 and 0.5 mg/dL, AST 510 and 65 U/L, Alk P 198 and 200 U/L], one required prednisone for rash, both recovered).
- Lang PG. Severe hypersensitivity reactions to allopurinol. South Med J. 1979;72:1361–8. [PubMed: 159491](Retrospective analysis of 20 cases of allopurinol hypersensitivity seen at 3 Atlanta hospitals 1973-78; 13 [65%] African-Americans, mean age 59 years, 11 with preexisting renal disease, 5 on thiazides; onset after 1-6 weeks often with rash, which was maculopapular [9], exfoliative [6] or toxic epidermal necrolysis [5]; 6 had liver involvement [bilirubin 1.4-13.8 mg/dL, AST 56-4000 U/L and Alk P 117-450 U/L], 9 had renal involvement, 4 died of complications of skin involvement and sepsis).
- Olsen H, Mørland J. Leverskader forårsaket av allopurinol. Tidsskr Nor Laegeforen. 1980;100(10):562–3. [Hepatic damages caused by allopurinol] Norwegian. [PubMed: 7385109](72 year old woman developed evidence of liver injury 3 months after starting allopurinol [ALT 365 U/L, Alk P 599 U/L], resolving within 1.5 weeks of stopping).
- Al-Kawas FH, Seeff LB, Berendson RA, Zimmerman HJ, Ishak KG. Allopurinol hepatotoxicity. Report of two cases and review of literature. Ann Intern Med. 1981;95:588–90. [PubMed: 7294548](Two African-American men developed fever and rash 3 and 4 weeks after starting allopurinol [bilirubin 1.5 and 5.4 mg/dL, ALT 650 and 1780 U/L, Alk P 193 and 248 U/L], recovery within 4-8 weeks of stopping, 1 given prednisone).
- Shah KA, Levin J, Rosen N, Greenwald E, Zumoff B. Allopurinol hepatotoxicity potentiated by tamoxifen. N Y State J Med. 1982;82:1745–6. [PubMed: 6960280](69 year old man on allopurinol for 12 years developed fever and Alk P and LDH elevations 1 day after starting tamoxifen for prostate cancer, fever and enzyme elevations, resolving within 3 days of stopping allopurinol; ALT and AST levels were elevated before tamoxifen therapy and did not change).
- Ramond MJ, Nouel O, Degott C, Lebrec D, Benhamou JP. Gastroenterol Clin Biol. 1982;6:138–42. [Allopurinol-induced hepatitis. Report of a case and review of the literature (author's transl)] [PubMed: 7060857](74 year old woman developed fever and jaundice one month after starting allopurinol with eosinophilia, [bilirubin 5.1 mg/dL, ALT 261 U/L, Alk P 6 times ULN], biopsy showing necrosis, inflammation and granulomas, resolving within 1-2 months of stopping).
- Falco D, Daniels RA, Conklin R. An unusual case of hypersensitivity vasculitis probably due to allopurinol. J Med Soc N J. 1982;79:409–12. [PubMed: 6954286](58 year old African American man with hypertension, congestive heart failure and renal insufficiency developed fatal, progressive vasculitis within days of restarting allopurinol; liver enzymes did not change and there was no eosinophilia).
- Raper R, Ibels L, Lauer C, Barnes P, Lunzer M. Fulminant hepatic failure due to allopurinol. Aust N Z J Med. 1984;14:63–5. [PubMed: 6590011](58 year old Chinese woman developed fever and exfoliative rash 3 weeks after starting allopurinol with eosinophilia, jaundice and fulminant course [bilirubin 12 rising to 30 mg/dL, ALT 1240 U/L, Alk P 1240 U/L]: Case 1).
- Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47–56. [PubMed: 6691361](Description of 6 patients with allopurinol toxicity and review of another 72 in the literature [latency 1-8 weeks in 90%, skin rash in 92%, fever 87%, eosinophilia 73%, hepatitis in 68%, renal worsening 85%, death 21%], also mentions that renal insufficiency was a strong risk factor and most patients were estimated to have high allopurinol levels, findings that led to recommendations for modification of dose based upon renal function).
- Ohsawa T, Ohtsubo M. Hepatitis associated with allopurinol. Drug Intell Clin Pharm. 1985;19:431–3. [PubMed: 4006738](66 year old woman developed severe hepatitis 10 days after starting allopurinol [peak bilirubin ~18 mg/dL, ALT 822 U/L, Alk P ~6 times ULN], resolving within 1 month, but recurrence of fever, AST elevation [97 U/L] and eosinophilia [16%] within hours of rechallenge with one dose).
- Mousson C, Justrabo E, Tanter Y, Chalopin JM, Rifle G. Nephrologie. 1986;7:199–203. [Acute granulomatous interstitial nephritis and hepatitis caused by drugs. Possible role of an allopurinol-furosemide combination] [PubMed: 3822042](58 year old woman with renal insufficiency developed fever and rash 6 weeks after starting allopurinol [bilirubin 2.2 mg/dL, ALT 56 U/L, Alk P 780 U/L], and worsening renal function and granulomas in both liver and kidney biopsies).
- Vanderstigel M, Zafrani ES, Lejonc JL, Schaeffer A, Portos JL. Allopurinol hypersensitivity syndrome as a cause of hepatic fibrin-ring granulomas. Gastroenterology. 1986;90:188–90. [PubMed: 3940244](39 year old man developed fever, rash and eosinophilia 4 weeks after starting allopurinol [bilirubin 1.2 mg/dL, ALT 167 U/L, Alk P 406 U/L], biopsy showed granulomas with central vacuole and fibrin ring, infectious causes ruled out).
- Singer JZ, Wallace SL. The allopurinol hypersensitivity syndrome. Unnecessary morbidity and mortality. Arthritis Rheum. 1986;29:82–7. [PubMed: 3947418](Review of 8 cases of allopurinol hypersensitivity in the literature; typically has onset after 2-12 weeks of therapy with fever, rash, eosinophilia and either renal [n=6] or liver [n=6] involvement, 3 died, 7 did not have strong indications for therapy; authors argue against use of allopurinol for asymptomatic hyperuricemia).
- Pewsner D, Bachmann C, Müller U. Schweiz Med Wochenschr. 1987;117:139–41. [Allopurinol-induced kidney failure with hepatitis and squamous dermatitis in pre-existing kidney insufficiency] [PubMed: 2950589](66 year old woman developed rash 6-8 weeks after starting allopurinol with jaundice and worsening renal function [peak bilirubin ~14.7 mg/dL, ALT 297 U/L, Alk P 855 U/L], resolving within 2 months of stopping).
- Olmos M, Guma C, Colombato LO, Lami G, Miyashiro R, Alvarez E. Acta Gastroenterol Latinoam. 1987;17:105–11. [Hepatic lesions induced by drugs. Report of 26 cases] Spanish. [PubMed: 3442185](Review of 2671 liver biopsies done in one Argentinian hospital between 1972-85 identified 26 with drug induced liver disease: 14 from birth control pills, 3 methyldopa, 2 carbon tetrachloride, 2 phenylbutazone, and 1 each from allopurinol [cholestatic hepatitis arising after 90 days], penicillin, chlorpromazine, indomethacin and ketoconazole).
- Stricker BH, Blok AP, Babany G, Benhamou JP. Fibrin ring granulomas and allopurinol. Gastroenterology. 1989;96:1199–203. [PubMed: 2925064](Found 6 cases of granulomas during allopurinol therapy reported to WHO database; onset of fever, rash, arthralgias and eosinophilia within 2-4 weeks of starting drug with biopsies showing ill-defined granuloma-like lesions, but not with fibrin rings).
- González Ramallo VJ, Rodríguez Gorostiza FJ, Muiño Míguez A, López de la Riva M. Rev Esp Enferm Apar Dig. 1989;75:317–8. [Severe cholestasis caused by allopurinol] Spanish. [PubMed: 2734478](74 year old man with mild renal insufficiency developed fever, rash and jaundice 20 days after starting allopurinol [bilirubin 11.1 mg/dL, ALT 107 U/L, Alk P ~3000 U/L]; biopsy showing cholestasis, resolution in 4 weeks of stopping).
- Tam S, Carroll W. Allopurinol hepatotoxicity. Am J Med. 1989;86:357–8. [PubMed: 2919623](Patient on unknown dose of allopurinol for uncertain period developed acute liver failure [initially, bilirubin 1.3 mg/dL, ALT 535 U/L, Alk P 331 U/L], allopurinol levels were ~25-fold elevated suggesting an inadvertent overdose).
- Chong RS, Ng HS, Teh LB, Ho JM. Hepatic granulomas-an experience over the last 8 years. Singapore Med J. 1990;31:422–6. [PubMed: 2259936](Retrospective review identified 20 cases of liver biopsies with granulomas seen over 8 year period, 1 was attributed to allopurinol hypersensitivity: abstract only).
- de Bayser L, Roblot P, Ramassamy A, Silvain C, Levillain P, Becq-Giraudon B. Hepatic fibrin-ring granulomas in giant cell arteritis. Gastroenterology. 1993;105:272–3. [PubMed: 8514044](70 year old man with giant cell arteritis and not on allopurinol had fibrin-ring granulomas in liver with high Alk P, but normal ALT and bilirubin).
- Arellano F, Sacristán JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993;27:337–43. [PubMed: 8453174](Review of 101 cases of allopurinol hypersensitivity syndrome reported in literature; mean age 57 years, 2/3rds men, fever 95%, rash 93%, leukocytosis 40%, eosinophilia 60%, AST elevation 88%, renal dysfunction common and may play role in pathogenesis).
- Berbegal J, Morera J, Andrada E, Navarro V, Lluch V, López-Benito I. Med Clin (Barc). 1994;102:178–80. [Syndrome of allopurinol hypersensitivity. Report of a new case and review of the Spanish literature] Spanish. [PubMed: 8127168](75 year old man developed rash and fever 2 weeks after starting allopurinol followed by facial edema and neuropathy with eosinophilia [bilirubin 0.7 mg/dL, ALT 51 U/L, Alk P 494 U/L], liver biopsy showing granulomas, and injury resolving within a few weeks of stopping).
- Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102:28S–30S. [PubMed: 8006430](Consensus of international group of dermatologists on criteria for Stevens Johnson syndrome [SJS] and toxic epidermal necrolysis [TEN] based upon pattern of erythema multiforme-like lesions and extent of dermal detachment).
- Lee SS, Lin HY, Wang SR, Tsai YY. Allopurinol hypersensitivity syndrome. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1994;27:140–7. [PubMed: 9747344]
- González U, Reyes E, Kershenovich J, Orozco-Topete RL. Rev Invest Clin. 1995;47:409–13. [Hypersensitivity syndrome caused by allopurinol. A case of massive hepatic necrosis] Spanish. [PubMed: 8584813](Abstract only: 72 year old woman developed fever and Stevens-Johnson syndrome 2 weeks after starting allopurinol, with subsequent fatal renal and hepatic failure).
- Paitel JF, Trechot P, Stockemer V, Dorvaux V, Lederlin P. Presse Med. 1995;24:460. [Acute liver disease during treatment with pipobroman and allopurinol] [PubMed: 7746823](41 year old woman taking allopurinol for 5 years, developed jaundice 2 months after starting pipobroman for polycythemia vera [bilirubin 11.8 mg/dL, ALT 33 times ULN, normal protime] and recovery in 4-6 months; unlikely due to allopurinol).
- Urban T, Maquarre E, Housset C, Chouaid C, Devin E, Lebeau B. Rev Mal Respir. 1995;12:314–6. [Allopurinol hypersensitivity. A possible cause of hepatitis and mucocutaneous eruptions in a patient undergoing antitubercular treatment] [PubMed: 7638430](39 year old man with tuberculosis developed fever and rash 2 weeks after starting allopurinol; initially with normal liver tests, but then developing lymphadenopathy, eosinophilia [bilirubin normal, ALT 413 U/L, Alk P 143 U/L], resolving rapidly after stopping allopurinol).
- Wade B, Klotz F, Debonne JM, Aubert M. Med Trop (Mars). 1995;55:186–7. [Fatal hepatitis due to allopurinol in Dakar] [PubMed: 7565008]
- Andrade RJ, de la Mata M, Lucena MI, López-Rubio F, Corrales MA. Gastroenterol Hepatol. 1997;20:353–6. [Severe acute hepatitis due to allopurinol in a patient with asymptomatic hyperuricemia and kidney failure. A review of the literature and an analysis of the risk factors] [PubMed: 9377233](Patient with mild renal failure developed fever, rash and eosinophilia 19 days after starting allopurinol [bilirubin rising from normal to 27.6 mg/dL, ALT 169 U/L, Alk P 472 U/L, with ascites and prothrombin index 47%], with slow but ultimately complete recovery).
- Jappe U, Franke I, Wendekamm U, Gollnick H. Hautarzt. 1998;49:126–30. [Allopurinol as an inducer of acute graft-versus-host-like drug reaction. Case report with review of the literature] German. [PubMed: 9551335]
- Pluim HJ, van Deuren M, Wetzels JFM. The allopurinol hypersensitivity syndrome. Neth J Med. 1998;52:107–10. [PubMed: 9599967](61 year old man developed fever, rash, eosinophilia, facial edema and lymphadenopathy 5 weeks after starting allopurinol, [bilirubin normal, ALT 115 U/L, Alk P 97 U/L] and renal insufficiency requiring high dose prednisolone; slow recovery).
- Kluger E. Ugeskr Laeger. 1998;160:1179–80. [Fatal outcome in allopurinol hypersensitivity syndrome] Danish. [PubMed: 9492630]
- Pereira S, Almeida J, Silva AO, Quintas M, Candeias O, Freitas F. Acta Med Port. 1998;11:1141–4. [Fatal liver necrosis due to allopurinol] Portuguese. [PubMed: 10192993]
- Khoo BP, Leow YH. A review of inpatients with adverse drug reactions to allopurinol. Singapore Med J. 2000;41:156–60. [PubMed: 11063179](13 patients with adverse reactions to allopurinol over 3 years, all with rash arising after 3-54 [mean=21] days, 10 with fever, 7 had elevated ALT [21-289 U/L], but none had jaundice and all recovered).
- Hammer B, Link A, Wagner A, Böhm M. Dtsch Med Wochenschr. 2001;126:1331–4. [Hypersensitivity syndrome during therapy with allopurinol in asymptomatic hyperuricemia with a fatal outcome] German. [PubMed: 11719858](86 year old woman developed high fever and severe rash progressing to toxic epidermal necrolysis arising 1 week after starting allopurinol for asymptomatic hyperuricemia, liver tests normal, but developed septicemia and multiorgan failure and died).
- Descamps V, Valance A, Edlinger C, Fillet AM, Crossin M, Lerun-Vignes B, Belatch S, et al. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch Dermatol. 2001;137:301–4. [PubMed: 11255328](Among 7 patients with drug reaction with eosinophilia and systemic symptoms [DRESS] syndrome, all had anti-HHV-6, 2 in rising titers, 4 with IgM, none had HHV-6 DNA; 5 cases from carbamazepine, 1 sulfasalazine and 1 ibuprofen).
- Vázquez-Mellado J, Morales EM, Pacheco-Tena C, Burgos-Vargas R. Relation between adverse events associated with allopurinol and renal function in patients with gout. Ann Rheum Dis. 2001;60:981–3. [PMC free article: PMC1753376] [PubMed: 11557658](Among 120 patients with gout treated with allopurinol, adverse events occurred in 5 [rash, hypersensitivity syndrome] including 3 of 52 [6%] receiving a creatinine adjusted dose and 2 of 68 [3%] receiving higher doses).
- Tjwa M, De Hertogh G, Neuville B, Roskams T, Nevens F, Van Steenbergen W. Hepatic fibrin-ring granulomas in granulomatous hepatitis: report of four cases and review of the literature. Acta Clin Belg. 2001;56:341–8. [PubMed: 11881318](4 patients with febrile illnesses were found to have fibrin-ring granulomas on liver biopsy; Q fever in 1, EBV in 1, CMV in 2, one of whom was also receiving allopurinol and thiazides chronically).
- ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother. 2002;36:130–47. [PubMed: 11816242](Review of drug induced causes of vasculitis, allopurinol ranking 4th after propylthiouracil, hydralazine and G-CSF; 16 cases reported; 12 men, ages 17-75 with rash, renal, hepatic and other organ involvement; high mortality rate).
- Chan YC, Tay YK, Ng SK. Allopurinol hypersensitivity syndrome and acute myocardial infarction-two case reports. Ann Acad Med Singapore. 2002;31:231–3. [PubMed: 11957564](Two patients with allopurinol hepatotoxicity treated with corticosteroids had myocardial infarctions several months after recovery; authors suggest a link between the two: abstract only).
- Muller P, Dubreil P, Mahé A, Lamaury I, Salzer B, Deloumeaux J, Strobel M. Drug hypersensitivity syndrome in a West-Indian population. Eur J Dermatol. 2003;13:478–81. [PubMed: 14693494](7 year prospective study in Guadeloupe of drug hypersensitivity syndrome; 28 cases found ~1/100,000 incidence, onset averaging 4 weeks, 2 deaths; 7 cases due to allopurinol, frequently given inappropriately, with possible racial predisposition).
- Descamps V, Mahe E, Houhou N, Abramowitz L, Rozenberg F, Ranger-Rogez S, Crickx B. Drug-induced hypersensitivity syndrome associated with Epstein-Barr virus infection. Br J Dermatol. 2003;148:1032–4. [PubMed: 12786838](Patient with rash and fever after 3 weeks of allopurinol with atypical lymphocytosis, eosinophilia, ALT ~250 U/L, Alk P 504 U/L, normal bilirubin and pancreatitis [amylase 1070 U/L], recovery starting 7-10 days after stopping drug; IgM anti-EBV and anti-HHV-6 were present).
- Mete N, Yilmaz F, Gulbahar O, Avdin A, Sin A, Kokuludaq A, Yuce G, Sebik F. Allopurinol hypersensitivity syndrome as a cause of hepatic centrilobular hemorrhagic necrosis. J Investig Allergol Clin Immunol. 2003;13:281–3. [PubMed: 14989119](41 year old developed fever, rash, jaundice and eosinophilia 3 weeks after starting allopurinol with fatal outcome, autopsy showing severe centrolobular necrosis: abstract only).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, but none were attributed to allopurinol).
- Dia D, Ba-Fall K, Bouldouyre M, Chevalier B, Debonne JM. Dakar Med. 2004;49:114–5. [DRESS syndrome to allopurinol: a case in Dakar] [PubMed: 15786619](47 year old man developed fever, rash and facial edema 3 months after starting allopurinol with eosinophilia and jaundice; rapid resolution with stopping drug: abstract only).
- Russmann S, Lauterburg B. Ther Umsch. 2004;61:575–7. [Life-threatening adverse effects of pharmacologic antihyperuricemic therapy] [PubMed: 15493119](Review of safety of allopurinol and newer xanthine oxidase inhibitors such as febuxostat and benzbromarone).
- Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Palo WA, Eustace D, Vernillet L, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–61. [PubMed: 16339094](762 patients at 112 North American centers received either allopurinol [300 mg/day] or febuxostat [80, 120 or 240 mg/day] for 52 weeks; reduction of uric acid to <6 mg/dL achieved in 53-62% of febuxostat- vs 21% of allopurinol-treated patients; rates of acute gout were similar; liver test abnormalities in 4-5% of febuxostat vs 4% of allopurinol recipients; most common cause of discontinuation [2.3%]).
- Gutiérrez-Macías A, Lizarralde-Palacios E, Martínez-Odriozola P, Miguel-De la Villa F. Fatal allopurinol hypersensitivity syndrome after treatment of asymptomatic hyperuricaemia. BMJ. 2005;331:623–4. [PMC free article: PMC1215560] [PubMed: 16166134](80 year old man developed fever, rash and jaundice with eosinophilia [16%] 6 weeks after starting allopurinol [bilirubin 31.2 mg/dL, ALT 328 U/L, Alk P 6567 U/L], progressing to hepatic failure and death; risk factors were preexisting renal insufficiency and furosemide use).
- Saxena R, Loghmanee F. Fatal drug reaction due to allopurinol therapy in a 72-year-old man. Arch Pathol Lab Med. 2005;129:e183–4. [PubMed: 16048416](72 year old man developed fever and severe rash 3 weeks after starting allopurinol, which progressed to generalized blistering and sloughing of skin and death from multiorgan failure).
- Markel A. Allopurinol-induced DRESS syndrome. Isr Med Assoc J. 2005;7:656–60. [PubMed: 16259349](Review of the hypersensitivity reactions to allopurinol including nephro- and hepatotoxicity).
- Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, Lin YL, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–9. [PMC free article: PMC554812] [PubMed: 15743917](The HLA-B*58:01 allele was present in all 51 [100%] Chinese patients with allopurinol associated severe cutaneous adverse reactions [SJS/TEN/DRESS], but only 15% of 135 allopurinol tolerant patients and 20% of healthy controls).
- Choi SH, Yang SH, Song YB, Kim HJ, Seo YT, Choi DS, Moon KH, et al. Allopurinol therapy and vanishing bile duct syndrome. Korean J Hepatol. 2005;11:80–5. [PubMed: 15788888](60 year old man developed fever, rash and eosinophilia 1 week after starting allopurinol, with progressive liver failure and death 7 weeks later [bilirubin 1.7 rising to 37.2 mg/dL, ALT 483 to 175 U/L, Alk P 140 to 579U/L], biopsy showing loss of bile ducts).
- Vital Durand D, Durieu I, Rousset H. Rev Med Interne. 2008;29:33–8. [Toxic or drug-induced granulomatous reactions] French. [PubMed: 18054121](Review of drug and toxin induced granulomatous reactions in various organs; in four recent case series, granulomas were found in 292 of 7754 biopsies [4%], of which 2-8% were attributable to drugs, but another 10-30% were "idiopathic" and possibly related; most common agent was allopurinol, but others implicated in single cases were chlorpropamide, phenothiazines, carbamazepine, methyldopa, baclofen, glibencamide, quinidine, metronidazole, and nitrofurantoin).
- Chiou CC, Yang LC, Hung SI, Chang YC, Kuo TT, Ho HC, Hu S, et al. Clinicopathological features and prognosis of drug rash with eosinophilia and systemic symptoms: a study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol. 2008;22:1044–9. [PubMed: 18627428](Over a 5 year period, 30 cases of DRESS syndrome were seen at a single referral center in Taiwan; 15 men and 15 women, ages 13 to 78 years, on drug for 3-60 days [mean=23 days], attributed to allopurinol in 11 [37%], carbamazepine in 6 [20%] and phenytoin, indomethacin and vancomycin in 2 each [7%]; liver test abnormalities in 87%, jaundice in 17% and 3 died [10%], but none of liver failure).
- Halevy S, Ghislain PD, Mockenhaupt M, Fagot JP, Bouwes Bavinck JN, Sidoroff A, Naldi L, et al. EuroSCAR Study Group. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008;58:25–32. [PubMed: 17919772](Among 379 patients with SJS/TEN, allopurinol was the most frequent cause [n=66: 17%], which was taken by only 2% of matched controls without severe cutaneous adverse events).
- Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, Sawada J, et al. JSAR research group. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9:1617–22. [PubMed: 19018717](Among 58 Japanese patients with SJS/TEN, none of 7 carbamazepine attributed cases had B*15:02, and only 4 of 10 allopurinol cases had B*58:01, the allele frequency being 20% in cases vs 0.6% in the population).
- Schumacher HR Jr, Becker MA, Wortmann RL, MacDonald PA, Hunt B, Streit J, Lademacher C, Joseph-Ridge N. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540–8. [PubMed: 18975369](Among 1072 patients with gout randomized to different treatments for 28 weeks, abnormal liver tests [=1.5 times ULN] occurred in 4-6% on febuxostat, 2% placebo, and 6% allopurinol).
- Shalom R, Rimbroth S, Rozenman D, Markel A. Allopurinol-induced recurrent DRESS syndrome: pathophysiology and treatment. Ren Fail. 2008;30:327–9. [PubMed: 18350453](65 year old man with mild renal insufficiency developed rash and fever, 1 week after starting allopurinol with eosinophilia [bilirubin 1.0 mg/dL, ALT 76 U/L] and responding to prednisone, but relapsing twice when prednisone was stopped with fever, rash, eosinophilia and more severe liver abnormalities [bilirubin 1.9 mg/dL, ALT 1784 U/L]).
- Khanlari B, Bodmer M, Terracciano L, Heim MH, Fluckiger U, Weisser M. Hepatitis with fibrin-ring granulomas. Infection. 2008;36:381–3. [PubMed: 17926000](66 year old woman developed fever and jaundice 4 months after starting allopurinol [bilirubin 1.2 mg/dL, ALT 89 U/L, Alk P 236 U/L] and ring granulomas on liver biopsy; corticosteroids led to worsening and repeat biopsy showed Leishmania: Kala-azar).
- Yoon JY, Min SY, Park JY, Hong SG, Park SJ, Paik SY, Park YM. Korean J Hepatol. 2008;14:97–101. [A case of allopurinol-induced granulomatous hepatitis with ductopenia and cholestasis] [PubMed: 18367862](69 year old man with renal failure developed rash and fever 2 weeks after starting allopurinol, liver biopsy showing spotty necrosis, cholestasis, ductopenia and granulomas, recovery with corticosteroids: abstract only).
- Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, Naldi L, et al. RegiSCAR study group. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18:99–107. [PubMed: 18192896](HLA-B genotyping of a cohort of patients with Stevens Johnson syndrome [SJS] due to medications found an association of B*58:01 with SJS due to allopurinol in 4 non-Europeans [100%] as well as 27 Europeans [55%] compared to controls [1.5%] and a slight association of B*3801 with SJS due to lamotrigine [24%] and sulfonamides [28%] compared to controls [4.3%]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, two were attributed to allopurinol).
- Tausche AK, Aringer M, Schroeder HE, Bornstein SR, Wunderlich C, Wozel G. The Janus faces of allopurinol-allopurinol hypersensitivity syndrome. Am J Med. 2008;121:e3–4. [PubMed: 18328291](69 year old woman developed rash 3 months after starting allopurinol [bilirubin not given, ALT 10 fold elevated], resolving with corticosteroid therapy).
- Eshki M, Allanore L, Musette P, Milpied B, Grange A, Guillaume JC, Chosidow O, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol. 2009;145:67–72. [PubMed: 19153346](Retrospective analysis of 15 patients with severe drug rash with eosinophilia and systemic symptoms [DRESS] syndrome from France; 2/3rds women, ages 15-71, onset average of 18 days, 4 due to allopurinol; severe manifestations including hepatitis [n=7], pneumonitis [10], renal failure [5], encephalitis [2], pancytopenia [2], heart failure [1]).
- Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY, Tiamkao S, Khunarkornsiri U, Chucherd P, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19:704–9. [PubMed: 19696695](Among 27 Thai patients with SJS/TEN due to allopurinol, all had HLA-B*58:01 compared to 7 of 54 [13%] allopurinol tolerant controls).
- Lindh J. Lakartidningen. 2009;106:2374–5. [Hepatic adverse effects of allopurinol] Swedish. [PubMed: 19848345](Commentary on response to ALT elevations occurring during allopurinol therapy mentions that the acute liver injury occurs in 0.2-0.4% of patients, typically in the first 2 months and with prominent immunoallergic features).
- Gyotoku E, Iwamoto T, Ochi M. Arerugi. 2009;58:560–6. [A fatal case of drug-induced hypersensitivity syndrome due to allopurinol] Japanese. [PubMed: 19487838](83 year old woman developed fever and disseminated rash 1 month after starting allopurinol with progressive liver failure and death: abstract only).
- Um SJ, Lee SK, Kim YH, Kim KH, Son CH, Roh MS, Lee MK. Clinical features of drug-induced hypersensitivity syndrome in 38 patients. J Investig Allergol Clin Immunol. 2010;20:556–62. [PubMed: 21313995](Among 38 patients with DRESS seen at a single referral center in Korea over a 5 year period, 20 were women, ages 24 to 80 years, onset after 3-105 days, all had liver involvement; 8 due to carbamazepine, 4 phenytoin, 3 lamotrigine, 2 phenobarbital and 2 allopurinol; 1 death from liver failure).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, one of which was attributed to allopurinol).
- Chen YC, Chiu HC, Chu CY. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373–9. [PubMed: 20713773](Among 60 cases of DRESS syndrome seen at a referral center in Taiwan over a 10 year period, the most common causes were allopurinol [31%], phenytoin [18%] and dapsone [17%]; 80% had hepatic manifestations; mean onset of allopurinol cases was 27 days, which was longer than for phenytoin [14 days] and others [19 days]).
- Somkrua R, Eickman EE, Saokaew S, Lohitnavy M, Chaiyakunapruk N. Association of HLA-B*5801 allele and allopurinol-induced Stevens Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. BMC Med Genet. 2011;12:118. [PMC free article: PMC3189112] [PubMed: 21906289](Systematic review of evidence linking HLA-B*58:01 to allopurinol associated Stevens Johnson syndrome [SJS] and toxic epidermal necrosis [TEN], in 4 studies with matched controls, 54 of 55 cases [98%], but only 74 of 678 controls [11%] had the HLA allele whereas in 5 studies using population control, the carrier frequency was 50 of 69 cases [72%] vs 171 of 3378 controls [5%]; the analysis combined European and Asian studies).
- Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH, Park HW, et al. Adverse Drug Reaction Research Group in Korea. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011;21:303–7. [PubMed: 21301380](Among 25 Korean patients with severe cutaneous adverse reactions to allopurinol, all except two [92%] had HLA-B*58:01 compared to 10.5% of 57 tolerant Korean patients).
- Génin E, Schumacher M, Roujeau JC, Naldi L, Liss Y, Kazma R, Sekula P, et al. Genome-wide association study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Europe. Orphanet J Rare Dis. 2011;6:52. [PMC free article: PMC3173287] [PubMed: 21801394](Genome wide association study on 424 cases of SJS/TEN from Europe identified 6 SNPs in the HLA region as being significant; best association being HLA-B*58:01 in 57 patients with allopurinol associated hypersensitivity).
- Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, Roujeau JC. The DRESS syndrome: a literature review. Am J Med. 2011;124:588–97. [PubMed: 21592453](Systematic review of literature on DRESS identified 172 cases due to 44 drugs, most frequently carbamazepine [27%] and allopurinol [11%], similar sex distribution, ages 0.5 to 80 years, onset after 0.5-16 weeks [mean=3.9], liver involvement 94%, eosinophilia 64%, mortality 5%).
- Natkunarajah J, Goolamali S, Craythorne E, Benton E, Smith C, Morris-Jones R, Wendon J, et al. Ten cases of drug reaction with eosinophilia and systemic symptoms (DRESS) treated with pulsed intravenous methylprednisolone. Eur J Dermatol. 2011;21:385–91. [PubMed: 21527371](Prospective study on use of methylprednisolone in 10 patients with DRESS, found rapid improvement in all but one who required a liver transplant).
- Jung JW, Song WJ, Kim YS, Joo KW, Lee KW, Kim SH, Park HW, et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant. 2011;26:3567–72. [PubMed: 21393610](Among 448 Korean patients with chronic renal dysfunction treated with allopurinol, 16 [3.6%] had a cutaneous adverse reaction, including 2 with SJS, 7 with allopurinol hypersensitivity syndrome [AHS] and 7 with maculopapular rash without systemic signs; HLA-B*58:01 was found in all 9 patients with SJS or AHS but in none with rash only and in only 9.5% who were tolerant to allopurinol).
- Lee MH, Stocker SL, Anderson J, Phillips EJ, Nolan D, Williams KM, Graham GG, et al. Initiating allopurinol therapy: do we need to know the patient's human leucocyte antigen status? Intern Med J. 2012;42:411–6. [PubMed: 21790926](HLA-B*58:01 was present in all 4 Asian patients with SJS due to allopurinol, but in only 2 of 8 Caucasians with SJS/hypersensitivity syndrome and in 0 of 12 with rash alone due to allopurinol).
- Tan SK, Tay YK. Profile and pattern of Stevens-Johnson syndrome and toxic epidermal necrolysis in a general hospital in Singapore: treatment outcomes. Acta Derm Venereol. 2012;92:62–6. [PubMed: 21710108](Among 28 cases of SJS/TEN seen at a single referral hospital in Singapore between 2004-2010, 26 were attributed to drugs, but only 2 were due to allopurinol).
- Pirmohamed M. Genetics and the potential for predictive tests in adverse drug reactions. Chem Immunol Allergy. 2012;97:18–31. [PubMed: 22613851](Brief review of the association of HLA alleles with cutaneous adverse events, focusing upon skin reactions to allopurinol, carbamazepine and abacavir and hepatotoxicity associated with flucloxacillin, lumiracoxib, lapatinib and ximelagatran).
- Yun J, Adam J, Yerly D, Pichler WJ. Human leukocyte antigens (HLA) associated drug hypersensitivity: consequences of drug binding to HLA. Allergy. 2012;67:1338–46. [PubMed: 22943588](Review of current understanding of the pathogenesis of the association of specific HLA alleles with drug induced hypersensitivity reactions, focusing upon abacavir, carbamazepine and allopurinol).
- Biagioni E, Busani S, Rinaldi L, Marietta M, Girardis M. Acute renal failure and liver necrosis associated to allopurinol therapy. Anaesth Intensive Care. 2012;40:190–1. [PubMed: 22313089]
- Botelho LF, Higashi VS, Padilha MH, Enokihara MM, Porro AM. DRESS: clinicopathological features of 10 cases from an University Hospital in São Paulo. An Bras Dermatol. 2012;87:703–7. [PubMed: 23044561](Among 10 patients with DRESS syndrome seen at a referral hospital in Brazil between 2005-2011, 6 were in men, ages 20 to 66 years, and onset occurred within 2-6 weeks of starting; phenytoin in 4, allopurinol in 2, or carbamazepine, diclofenac, or both in 1 each; 2 died of acute liver failure).
- Yaylacı S, Demir MV, Temiz T, Tamer A, Uslan MI. Allopurinol-induced DRESS syndrome. Indian J Pharmacol. 2012;44:412–4. [PMC free article: PMC3371471] [PubMed: 22701258](70 year old Turkish man with hypertension and renal disease developed fever, rash and jaundice 3 months after starting allopurinol [bilirubin 18.9 mg/dL, ALT 429 U/L, Alk P 773 U/L, INR 1.4], with progressive renal and liver failure and death within 24 days of onset).
- Cao ZH, Wei ZY, Zhu QY, Zhang JY, Yang L, Qin SY, Shao LY, et al. HLA-B*58:01 allele is associated with augmented risk for both mild and severe cutaneous adverse reactions induced by allopurinol in Han Chinese. Pharmacogenomics. 2012;13:1193–201. [PubMed: 22909208](Among 38 Chinese patients with severe cutaneous reactions from allopurinol, all had HLA-B*58:01 [5 were homozygous] compared to 11% of matched controls [none of whom were homozygous] and 14% of population controls).
- Stamp LK, Taylor WJ, Jones PB, Dockerty JL, Drake J, Frampton C, Dalbeth N. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529–36. [PubMed: 22488501](Among 54 cases of allopurinol hypersensitivity syndrome identified over a 12 year period in New Zealand, 56% were men, ages 24 to 87 years, mean onset in 30 days, 3 were fatal [6%]; compared to controls, cases were more likely to start at a higher dose when corrected for creatinine).
- Chiu ML, Hu M, Ng MH, Yeung CK, Chan JC, Chang MM, Cheng SH, Li L, Tomlinson B. Association between HLA-B*58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. Br J Dermatol. 2012;167:44–9. [PubMed: 22348415](Among 20 Chinese patients from Hong Kong with severe cutaneous reactions to allopurinol, 19 [95%] had HLA B*58:01 compared to 13% of 30 allopurinol tolerant controls).
- Maekawa K, Nishikawa J, Kaniwa N, Sugiyama E, Koizumi T, Kurose K, Tohkin M, et al. Development of a rapid and inexpensive assay for detecting a surrogate genetic polymorphism of HLA-B*58:01: a partially predictive but useful biomarker for allopurinol-related Stevens-Johnson syndrome/toxic epidermal necrolysis in Japanese. Drug Metab Pharmacokinet. 2012;27:447–50. [PubMed: 22277675](Description of a polymerase chain reaction based assay specifically designed to detect HLA-B*58:01 polymorphisms).
- Profaizer T, Eckels D. HLA alleles and drug hypersensitivity reactions. Int J Immunogenet. 2012;39:99–105. [PubMed: 22136512](Review of the HLA associations of drug hypersensitivity reactions to abacavir, allopurinol and carbamazepine).
- Bharadwaj M, Illing P, Theodossis A, Purcell AW, Rossjohn J, McCluskey J. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol. 2012;52:401–31. [PubMed: 22017685](Review of the molecular pathogenesis of T cell mediated hypersensitivity reactions to small molecule drugs, focusing upon class I HLA B*57:01 associations with abacavir and flucloxacillin], B*58:01 with allopurinol, and B*15:02 with carbamazepine and possibly phenytoin; and type II HLA associations with lapatinib and ximelagatran).
- Wongkitisophon P, Chanprapaph K, Rattanakaemakorn P, Vachiramon V. Six-year retrospective review of drug reaction with eosinophilia and systemic symptoms. Acta Derm Venereol. 2012;92:200–5. [PubMed: 22002792](Among 27 patients with DRESS syndrome seen at a single center in Thailand, 14 were men and ages ranged from 23 to 81 years; 96% of cases had hepatic manifestations, 70% eosinophilia, 19% atypical lymphocytosis; common causes were phenytoin [33%] allopurinol [15%], nevirapine [15%]; liver injury resolved in 20-65 days except in 1 patient who died [4%]).
- Bollaert M, Jeulin H, Waton J, Gastin I, Tréchot P, Rabaud C, Schmutz JL, Barbaud A. Ann Dermatol Venereol. 2012;139:15–22. [Six cases of spring DRESS] [PubMed: 22225738](Six cases of DRESS syndrome presented at a single hospital during a one month period, suggesting an epidemic, although each case was due to a different drug [allopurinol, carbamazepine, amoxicillin, TMP/SMZ, and others], and all had evidence of reactivation or primary infection with a herpes virus).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, Presentation and Outcomes in Patients with Drug-Induced Liver Injury in the General Population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to allopurinol).
- Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, Sidoroff A, et al. The RegiSCAR study group. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80. [PubMed: 23855313](Among 117 cases of DRESS enrolled in a European prospective database over a 6 year period, 18% were due to allopurinol, 75% had liver involvement and only 2 died [2%]).
- Paisansinsup T, Breitenstein MK, Schousboe JT. Association between adverse reactions to allopurinol and exposures to high maintenance doses: implications for management of patients using allopurinol. J Clin Rheumatol. 2013;19:180–6. [PubMed: 23669799](Analysis of electronic medical records of a multispecialty group practice between 2004-2011 identified 2946 patients who were treated with allopurinol and had a serum creatinine level; 48 [2%] had an adverse drug reaction [mostly rash] and 2 had SJS; no association was found between these reactions and allopurinol dose even after adjustment for renal function).
- Ramasamy SN, Korb-Wells CS, Kannangara DR, Smith MW, Wang N, Roberts DM, Graham GG, et al. Allopurinol hypersensitivity: a systematic review of all published cases, 1950-2012. Drug Saf. 2013;36:953–80. [PubMed: 23873481](Systematic review of literature on allopurinol hypersensitivity syndrome identified 901 patients in 320 publications, including 58% men, 73% Asians, median onset in 3 weeks, mortality rate 14%; among those with liver involvement, mean bilirubin 6.4 mg/dL, ALT 358 U/L, Alk P 518 U/L; 166 of 167 Asian patients tested were HLA-B*58:01 positive).
- Kwok J, Kwong KM. Detection of HLA-B*58:01, the susceptible allele for allopurinol-induced hypersensitivity, by loop-mediated isothermal amplification. Br J Dermatol. 2013;168:526–32. [PubMed: 23066948](Description of development of a loop mediated isothermal amplification method for identifying DNA of HLA-B*58:01 that can be used clinically and is faster and easier than standard genotyping methods).
- Kim SC, Newcomb C, Margolis D, Roy J, Hennessy S. Severe cutaneous reactions requiring hospitalization in allopurinol initiators: a population-based cohort study. Arthritis Care Res (Hoboken). 2013;65:578–84. [PMC free article: PMC3502684] [PubMed: 22899369](Using electronic records of 5 large Medicaid programs of approximately 13 million enrollees, 90,358 patients were started on allopurinol and were followed, among whom 45 were subsequently hospitalized for a severe cutaneous reaction [0.69 per 1000 patient years] and 12 died; rates that were far higher than in non-allopurinol users [0.04 per 1000 patient years).
- Ryu HJ, Song R, Kim HW, Kim JH, Lee EY, Lee YJ, Song YW, Lee EB. Clinical risk factors for adverse events in allopurinol users. J Clin Pharmacol. 2013;53:211–6. [PubMed: 23436266](Case control study of 94 patients with adverse event [rash, GI upset, hypersensitivity syndrome, severe, neuropathy] due to allopurinol and 378 tolerant controls found risk factors to be renal disease and use of colchicine or statins).
- Lee MH, Stocker SL, Williams KM, Day RO. HLA-B*5801 should be used to screen for risk of Stevens-Johnson syndrome in family members of Han Chinese patients commencing allopurinol therapy. J Rheumatol. 2013;40:96–7. [PubMed: 23280169](Letter describing Chinese man who developed SJS after allopurinol exposure and tested positive for HLA-B*58:01 who had a brother who tolerated allopurinol, but was B*58:01 negative).
- Walsh S, Diaz-Cano S, Higgins E, Morris-Jones R, Bashir S, Bernal W, Creamer D. Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168:391–401. [PubMed: 23034060](Among 27 patients with DRESS presenting at a single UK referral center over a 6 year period, 2 [7%] were due to allopurinol, 6 phenytoin, 5 carbamazepine; the mortality rate was 11% and severe cases were more likely to have erythema multiforme like lesions by histology).
- Choi HG, Byun J, Moon CH, Yoon JH, Yang KY, Park SC, Han CJ. Allopurinol-induced DRESS syndrome mimicking biliary obstruction. Clin Mol Hepatol. 2014;20:71–5. [PMC free article: PMC3992333] [PubMed: 24757661](84 year old man developed fever, rash and jaundice 2 months after starting allopurinol [bilirubin 6.6 rising to 27.1 mg/dL, ALT 343 U/L, Alk P 210 U/L, eosinophils 14%], with progressive worsening and sudden death from a myocardial infarction).
- Sultan SJ, Sameem F, Ashraf M. Drug reaction with eosinophilia and systemic symptoms: manifestations, treatment, and outcome in 17 patients. Int J Dermatol. 2015;54(5):537–42. [PubMed: 24738653](Among 17 patients with DRESS syndrome seen at a single medical center in India over a 4 year period, causes were anticonvulsants [65%], dapsone, vancomycin, leflunomide, nitrofurantoin and allopurinol).
- Daly AK. Human leukocyte antigen (HLA) pharmacogenomic tests: potential and pitfalls. Curr Drug Metab. 2014;15:196–201. [PubMed: 24694233](Discussion of the shortcomings of use of HLA associations in clinical decisions to use drugs associated with severe hypersensitivity reactions including allopurinol).
- Karlin E, Phillips E. Genotyping for severe drug hypersensitivity. Curr Allergy Asthma Rep. 2014;14:418. [PMC free article: PMC4014135] [PubMed: 24429903](Review of the genetic associations and potential clinical applications of genotyping to prevent serious drug adverse reactions, including testing for HLA-B*58:01 to assess the risk of SJS from allopurinol, particularly in Asian populations).
- Thankachen J, Agarwal V. Challenges in diagnosis, management, and treatment of allopurinol-induced DRESS syndrome: case report and literature review. Am J Ther. 2015;22(3):e77–83. [PubMed: 24451301](62 year old African American woman developed fever, rash and confusion 3 weeks after a 2 day course of allopurinol [bilirubin not given, ALT 116 U/L, Alk P 198 U/L, eosinophils 16%], rash considered typical of DRESS, responding to corticosteroids, and resolving within 3 months of onset).
- Nam YH, Park MR, Nam HJ, Lee SK, Kim KH, Roh MS, Um SJ, Son CH. Drug reaction with eosinophilia and systemic symptoms syndrome is not uncommon and shows better clinical outcome than generally recognised. Allergol Immunopathol (Madr). 2015;43(1):19–24. [PubMed: 24388810](Among 45 patients with DRESS seen over a 2 year period at a single Korean referral center, all had fever, rash and eosinophilia, 87% had liver involvement and 1 died of liver failure and 1 of sepsis; common causes included cephalosporins [29%], penicillins [9%], anticonvulsants [27%], NSAIDs [9%] and allopurinol [7%]).
- Yang MS, Kang MG, Jung JW, Song WJ, Kang HR, Cho SH, Min KU. Clinical features and prognostic factors in severe cutaneous drug reactions. Int Arch Allergy Immunol. 2013;162:346–54. [PubMed: 24193402](Allopurinol accounted for 17% of 41 cases of SJS/TEN and 31% of cases of DRESS syndrome; ALT elevations occurred in 46% of SJS/TEN and 75% of DRESS cases).
- Lee T, Lee YS, Yoon SY, Kim S, Bae YJ, Kwon HS, Cho YS, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol. 2013;69:407–15. [PubMed: 23632341](Among 136 Korean patients with drug hypersensitivity reactions, 61 had liver dysfunction, 29 with DRESS and 11 with SJS/TEN, and allopurinol was the most common cause in SJS/TEN cases).
- Gonçalo M, Coutinho I, Teixeira V, Gameiro AR, Brites MM, Nunes R, Martinho A. HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br J Dermatol. 2013;169:660–5. [PubMed: 23600531](Among 6 Portuguese patients with SJS/TEN and 19 with DRESS due to allopurinol, HLA-B*58:01 was present in 16 [64%] compared to 4% of 28 allopurinol tolerant persons and 2% of normal controls; rates similar to those reported in other European populations).
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15–23. [PubMed: 23866082](Review of the definition, epidemiology, causes, clinical features, pathology, pathogenesis and management of DRESS syndrome; allopurinol being the second most common cause).
- Limpawattana P, Choonhakarn C, Kongbunkiat K. Clinical profiles of Stevens-Johnson syndrome among Thai patients. J Dermatol. 2014;41(7):634–7. [PubMed: 24815085](Among 45 patients with SJS seen at a single referral center in Thailand over a 10 year period, liver injury occurred in 67%, but was usually mild and allopurinol accounted for 13% of cases, including 6 of 13 cases in patients over the age of 65).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to allopurinol).
- Miederer SE, Miederer KO. Dtsch Med Wochenschr. 2014;139:2537–40. [Allopurinol hypersensitivity syndrome: Liver transplantation after treatment of asymptomatic hyperuricaemia] [PubMed: 25423467](41 year old man developed fever, rash and liver test abnormalities 1 month after starting allopurinol [bilirubin rising to 54 mg/dL, ALT 548 U/L, Alk P 1268 U/L], and undergoing successful liver transplantation).
- Jung JW, Kim DK, Park HW, Oh KH, Joo KW, Kim YS, Ahn C. Leet al. An effective strategy to prevent allopurinol-induced hypersensitivity by HLA typing. Genet Med. 2015;17:807–14. [PubMed: 25634024](Among 401 Korean patients with renal disease planning to start allopurinol, 46 had HLA-B*58:01 and received a tolerance induction protocol [n=30] or another medication [n=16], and none developed serious cutaneous reactions, nor did any of the 355 who tested negative for this HLA allele).
- Ko TM, Tsai CY, Chen SY, Chen KS, Yu KH, Chu CS, Huang CM, et al. Taiwan Allopurinol-SCAR Consortium. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. 2015;351:h4848. [PMC free article: PMC4579807] [PubMed: 26399967](Among 2926 Taiwanese patients planning to start allopurinol, 571 had HLA-B*58:01 and were advised not to take it, of whom none developed rash; among the 2173 without this HLA allele who took allopurinol, 97 [3%] had mild rash, but none had a severe cutaneous reaction).
- Yip VL, Alfirevic A, Pirmohamed M. Genetics of immune-mediated adverse drug reactions: a comprehensive and clinical review. Clin Rev Allergy Immunol. 2015;48:165–75. [PubMed: 24777842](Review of the genetics of immune mediated drug reactions focusing upon skin injury from abacavir [HLA-B*57:01], carbamazepine [B*15:02, A*31:01], and allopurinol [B*58:01] and hepatotoxicity from amoxicillin/ clavulanate [several], flucloxacillin [B*57:01], ximelagatran, lumiracoxib, lapatinib, ticlopidine and nevirapine).
- Imai H, Kamei H, Onishi Y, Yamada K, Ishizu Y, Ishigami M, Goto H, Ogura Y. Successful living-donor liver transplantation for cholestatic liver failure induced by allopurinol: case report. Transplant Proc. 2015;47:2778–81. [PubMed: 26680093](39 year old Japanese man developed severe cholestatic liver injury 2 months after starting allopurinol for hyperuricemia [bilirubin rising to 60.9 mg/dL, INR 3.5], undergoing successful living donor liver transplantation 4 months later).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 7 cases [0.9%] were attributed to allopurinol, 3 of which were severe and one fatal).
- Sultan SJ, Sameem F, Ashraf M. Drug reaction with eosinophilia and systemic symptoms: manifestations, treatment, and outcome in 17 patients. Int J Dermatol. 2015;54:537–42. [PubMed: 24738653](Among 17 patients with DRESS syndrome seen at a referral center in India over a 4 year period, most cases [65%] were due to aromatic anticonvulsants, 2 to allopurinol and 1 each for vancomycin, leflunomide, dapsone and nitrofurantoin, with ALT elevations above 100 U/L in all 17, hyperbilirubinemia in 65% and liver failure in one).
- Park HJ, Kim SR, Leem DW, Moon IJ, Koh BS, Park KH, Park JW, et al. Clinical features of and genetic predisposition to drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a single Korean tertiary institution patients-investigating the relation between the HLA -B*4403 allele and lamotrigine. Eur J Clin Pharmacol. 2015;71:35–41. [PubMed: 25327504](Among 30 patients with SJS/TEN seen at a single Korean medical center between 2010 and 2014, 63% had accompanying liver injury and 2 died; allopurinol was the single most common cause [n=7: 27%], 5 cases of which had HLA-B*58:01 vs 1 of the 23 others, mean latency was 20 days).
- Civardi G, Zanlari L, Bassi E, Zangrandi A, Maria Cesinaro A, Nosseir S, De Maria N. Life threatening, allopurinol-related Dress syndrome as a rare cause of fever of unknown origin. Intern Med. 2015;54:2073–7. [PubMed: 26278306](38 year old woman developed fever, rash and eosinophilia 2-3 weeks after starting allopurinol for hyperuricemia, followed by severe liver and renal dysfunction but with ultimate full recovery).
- Polimeni G, Cardillo R, Garaffo E, Giardina C, Macrì R, Sirna V, Guarneri C, Arcoraci V. Allopurinol-induced Sweet's syndrome. Int J Immunopathol Pharmacol. 2016;29:329–32. [PMC free article: PMC5806712] [PubMed: 26684631](87 year old woman developed “Sweet’s” syndrome 8 days after starting allopurinol for hyperuricemia [6.2 mg/dL] with fever, rash, pus-filled skin blisters, conjunctivitis, joint pains and leukocytosis [bilirubin 1.3 mg/dL, ALT and Alk P not provided, GGT “doubling”], responding to stopping allopurinol and systemic corticosteroids).
- Hiransuthikul A, Rattananupong T, Klaewsongkram J, Rerknimitr P, Pongprutthipan M, Ruxrungtham K. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS): 11 years retrospective study in Thailand. Allergol Int. 2016;65:432–8. [PubMed: 27134114](Among 52 patients with DRESS syndrome seen at a referral hospital in Thailand between 2004 and 2014, 12 cases were due to phenytoin, 9 nevirapine, 8 allopurinol and 7 amoxicillin/clavulanate; the allopurinol cases had a mean age of 75 years, latency 30 days, 88% with eosinophilia, 100% with liver and 63% with kidney involvement, no deaths but 58% received systemic corticosteroids).
- Pinto Gouveia M, Gameiro A, Coutinho I, Pereira N, Cardoso JC, Gonçalo M. Overlap between maculopapular exanthema and drug reaction with eosinophilia and systemic symptoms among cutaneous adverse drug reactions in a dermatology ward. Br J Dermatol. 2016;175:1274–83. [PubMed: 27128886](Among 132 patients with cutaneous adverse drug reactions admitted to a dermatology ward in a Portuguese referral center between 2010 and 2015, 37 met criteria for DRESS syndrome whereas 28 had a maculopapular exanthem, 7 had SJS and 55 other dermatoses; allopurinol was the most frequent cause of DRESS [n=15: 40%], and both eosinophilia and liver involvement were frequent [both 78%] and among allopurinol cases, HLA-B*58:01 was present in 7 of 11 [64%] with DRESS and 2 of 5 [40%] with other maculopapular exanthema).
- Stamp LK, Chapman PT, Barclay ML, Horne A, Frampton C, Tan P, Drake J, et al. A randomised controlled trial of the efficacy and safety of allopurinol dose escalation to achieve target serum urate in people with gout. Ann Rheum Dis. 2017;76:1522–8. [PubMed: 28314755](Among 183 patients with gout on allopurinol but with serum uric acid levels above 6 mg/dL who were either maintained on the same dose or had dose increases, serum uric acid levels decreased more with higher doses, but there were no differences in flares of gout, and side effects of pruritus and rash and GGT elevations were more frequent with dose escalation but no patient developed clinically apparent liver injury).
- Waseem H, Inayat F, Abduraimova M, Kamholz S. Allopurinol-induced drug reaction with eosinophilia and systemic symptoms syndrome: a cause of acalculous cholecystitis? Cureus. 2017;9:e1569. [PMC free article: PMC5642814] [PubMed: 29057181](71 year old Chinese woman developed rash and fever within days of starting allopurinol with two recurrences after restarting for 1-2 days [eosinophils 17%, bilirubin 2.2 mg/dL, ALT 56 U/L, Alk P 100] thought to also have acalculous cholecystitis, rash and fever responding rapidly to systemic corticosteroids).
- Iqbal U, Siddiqui HU, Anwar H, Chaudhary A, Quadri AA. Allopurinol-induced granulomatous hepatitis: a case report and review of literature. J Investig Med High Impact Case Rep. 2017;5:2324709617728302. [PMC free article: PMC5644365] [PubMed: 29082266](83 year old man was found to have abnormal liver tests having been taking allopurinol, losartan and statins for years [bilirubin 0.7 mg/dL, ALT 81 U/L, Alk P 645 U/L], liver biopsy showing granulomatous hepatitis and liver tests improving but still abnormal 3 months later [ALT 44 U/L, Alk P 305 U/L]).
- El Kahi C, Martinet V, Praet JP, Pepersack T. Un cas rare d’hépatotoxicité en gériatrie. Rev Med Brux. 2018;39:164–5. [A rare case of hepatotoxicity in geriatrics] French. [PubMed: 29964389](Ordered: 12.26.2020).
- Wu X, Yang F, Chen S, Xiong H, Zhu Q, Gao X, Xing Q, et al. Clinical, viral and genetic characteristics of drug reaction with eosinophilia and systemic symptoms (DRESS) in Shanghai, China. Acta Derm Venereol. 2018;98:401–5. [PubMed: 29242946](Among 52 patients with DRESS syndrome seen at a referral hospital in Shanghai, China between 2011 and 2016, allopurinol was the most frequently implicated agent [n=18: 35%], followed by sulfasalazine [n=11: 21%] and carbamazepine [n=5; 10%]; rash was present in all patients, fever in 75%, eosinophilia in 81% and liver involvement in 83%; all allopurinol cases had HLA-B*58:01 and 2 had B*31:02, while sulfasalazine cases were linked to B*13:01 and carbamazepine to A*31:01 and B*13:01).
- Jung JW, Kim JY, Park IW, Choi BW, Kang HR. Genetic markers of severe cutaneous adverse reactions. Korean J Intern Med. 2018;33:867–75. [PMC free article: PMC6129640] [PubMed: 29921043](Review of genetic associations with severe cutaneous reactions such as SJS and DRESS syndrome including the close association of allopurinol reactions and B*58:01; nevertheless, estimates from Korea suggest that only 18% of H*58:01 carriers develop allopurinol hypersensitivity reactions, indicating that other factors must play a role in the etiology).
- Batista M, Cardoso JC, Oliveira P, Gonçalo M. Allopurinol-induced DRESS syndrome presented as a cholecystitis-like acute abdomen and aggravated by antibiotics. BMJ Case Rep. 2018;2018:bcr2018226023. [PMC free article: PMC6059224] [PubMed: 30042109](48 year old Caucasian man developed fever and right upper quadrant pain 4 weeks after starting allopurinol for gout [bilirubin not provided, ALT 575 U/L, Alk P 206 U/L, creatinine 1.24 mg/dL] leading to laparoscopic cholecystectomy which revealed a perforated gall bladder without stones; postoperatively he developed rash, eosinophilia and worsening liver and renal abnormalities, ultimately responding to systemic corticosteroids).
- Wang YH, Chen CB, Tassaneeyakul W, Saito Y, Aihara M, Choon SE, Lee HY, et al. Asian Severe Cutaneous Adverse Reaction Consortium. The medication risk of Stevens-Johnson Syndrome and toxic epidermal necrolysis in Asians: The major drug causality and comparison with the US FDA label. Clin Pharmacol Ther. 2019;105:112–20. [PubMed: 29569740](Analysis of registration databases from multiple Asian countries between 1998 to 2017 identified 2018 cases of SJS/TEN, the most common causes being carbamazepine [26%], allopurinol [20%], phenytoin [13%], lamotrigine [10%], sulfamethoxazole [8%], aminopenicillins [3%], fluoroquinolones [2.5%], cephalosporins [2.3%], phenobarbital [2%] and multiple others).
- Nguyen KD, Tran TN, Nguyen MT, Nguyen HA, Nguyen HA Jr, Vu DH, Nguyen VD, et al. Drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Vietnamese spontaneous adverse drug reaction database: a subgroup approach to disproportionality analysis. J Clin Pharm Ther. 2019;44:69–77. [PubMed: 30129156](Among 136 spontaneous reports of SJS/TEN to a national Vietnamese registry between 2010 and 2015, 60% were male, mean age 42 years and 92% associated with a medication, most commonly carbamazepine [n=25], allopurinol [n=15] herbal medications [n=7], colchicine [n=4], valproate [n=3] and meloxicam [n=3]).
- Devarbhavi H, Raj S. Drug-induced liver injury with skin reactions: Drugs and host risk factors, clinical phenotypes and prognosis. Liver Int. 2019;39:802–11. [PubMed: 30515930](Review of drugs that cause liver injury with concurrent skin reactions such as DRESS syndrome and SJS/TEN mentions that allopurinol is one of the most common causes and is associated with HLA-B*58:01 in Han Chinese, this allele being rare in Europeans, Japanese and Africans).
- Kang MG, Sohn KH, Kang DY, Park HK, Yang MS, Lee JY, Kang HR. Analysis of individual case safety reports of severe cutaneous adverse reactions in Korea. Yonsei Med J. 2019;60:208–15. [PMC free article: PMC6342723] [PubMed: 30666843](Analysis of reports of serious cutaneous reactions to the Korean registry between 1988 and 2013 identified 755 cases [508 SJS/TEN, 247 DRESS], allopurinol being the most common cause of both [10% and 11%] followed by carbamazepine and others most of which were linked to both forms at similar rates; among 20 deaths, 2 were attributed to allopurinol [both SJS/TEN]).
- Drugs for gout. Med Lett Drugs Ther. 2019;61(1567):33–7. [PubMed: 30845096](Concise summary of the mechanism of action, clinical efficacy, safety and costs of drugs for gout discusses allopurinol as an agent to prevent flares that is widely used, often in inadequate doses, and with potentially serious side effects of severe cutaneous reactions, aminotransferase elevations and vasculitis and that Asian patients should be screened for HLA-A*58:01, a risk factor for SJS).
- Sim DW, Yu JE, Jeong J, Jung JW, Kang HR, Kang DY, Ye YM, et al. Korean Severe Cutaneous Adverse Reactions Consortium. Variation of clinical manifestations according to culprit drugs in DRESS syndrome. Pharmacoepidemiol Drug Saf. 2019;28:840–8. [PubMed: 31044478](Among 123 cases of DRESS syndrome identified in a Korean registry for severe cutaneous reactions between 2011 and 2016, allopurinol accounted for 28 cases [23%], carbamazepine 22 [18%], antituberculosis agents 19 [15%], vancomycin 18 [15%], cephalosporins 15 [12%], dapsone 11 [9%], and NSAIDS 10 [8%]: allopurinol cases had a mean age of 56 years, 39% were women, 93% had eosinophilia, 86% had liver involvement and 79% renal, the mean latency was 30 days and there was only one death).
- Park HJ, Yun J, Kang DY, Park JW, Koh YI, Kim S, Kim SH, et al. Drug Allergy Work Group of KAAACI. Unique clinical characteristics and prognosis of allopurinol-induced severe cutaneous adverse reactions. J Allergy Clin Immunol Pract. 2019;7:2739–2749.e3. [PubMed: 31201937](Among 106 subjects with a severe cutaneous reaction due to allopurinol reported to a national Korean registry between 2010 and 2015, those with SJS/TEN were older [71 vs 56 years], had a shorter latency [19 vs 29 days], and a higher mortality rate [17.6% vs 3.7%]).
- Kreijne JE, de Veer RC, de Boer NK, Dijkstra G, West R, Moorsel SAW, de Jong DJ, et al. of the Dutch Initiative on Crohn, Colitis (ICC). Real-life study of safety of thiopurine-allopurinol combination therapy in inflammatory bowel disease: myelotoxicity and hepatotoxicity rarely affect maintenance treatment. Aliment Pharmacol Ther. 2019;50:407–15. [PubMed: 31359480](Among 221 patients with inflammatory bowel disease treated with low dose thiopurine combined with allopurinol, hepatotoxicity resolved in 86% of those with liver injury on thiopurine monotherapy but rose de novo in 31% of treated subjects, but was mostly mild [91%: ALT less than 3 times ULN] and self-limited in course, but was severe [ALT >5 times ULN] in 9 subjects and led to discontinuation in 2).
- Zhang F, Liu Z, Jiang L, Zhang H, Zhao D, Li Y, Zou H, Wang X, Li X, Shi B, Xu J, Yang H, Hu S, Qu S. A randomized, double-blind, non-inferiority study of febuxostat versus allopurinol in hyperuricemic Chinese subjects with or without gout. Rheumatol Ther. 2019;6:543–57. [PMC free article: PMC6858416] [PubMed: 31531831](Among 472 patients with hyperuricemia treated with titrated up doses of febuxostat [40 or 80 mg] or allopurinol [300 mg] for 24 weeks, response rates [uric acid 6.0 mg/dL or less] were highest with febuxostat 80 mg [63%] vs 40 mg [43%] and allopurinol[49%], while adverse event rates were similar including ALT elevations [6.5% vs 6.2% and 7.1%]).
- Yang MS, Lee JY, Kim J, Kim GW, Kim BK, Kim JY, Park HW, et al. Searching for the culprit drugs for Stevens-Johnson syndrome and toxic epidermal necrolysis from a nationwide claim database in Korea. J Allergy Clin Immunol Pract. 2020;8:690–695.e2. [PubMed: 31614216](Search using a Korean national health claims database identified 138 patients with SJS/TEN and the most commonly implicated agent was allopurinol [n=19]).
- Myers CM, Miller JJ, Davis WD. Allopurinol-induced drug reaction with eosinophilia and systemic symptoms: a case report. Adv Emerg Nurs J. 2020;42:108–18. [PubMed: 32358426](68 year old Chinese man developed rash and fever 3 weeks after starting allopurinol [100 mg daily] for asymptomatic hyperuricemia with eosinophilia and both renal and hepatic manifestations [bilirubin, ALT and Alk P not provided], responding ultimately to systemic corticosteroids).
- Hakim C, Melitas C, Nguyen E, Ngo K. Atypical manifestation of DRESS syndrome. Case Rep Gastrointest Med. 2020;2020:6863582. [PMC free article: PMC7174907] [PubMed: 32328318](52 year old African American man developed fever one week after completing a course of oral vancomycin and receiving allopurinol [unspecified duration and dose] during which he developed a rash and was treated with prednisone, which with tapering was followed by worsening fever and elevations in liver and kidney tests [bilirubin 0.6 mg/dL, ALT 1117 U/L, Alk P 169 U/L, creatinine 6.4 mg/dL, eosinophilia 13%], with subsequent spontaneous improvement).
- Hasegawa A, Abe R. Recent advances in managing and understanding Stevens-Johnson syndrome and toxic epidermal necrolysis. F1000Res 2020; 9: F1000 Faculty Rev-612. [PMC free article: PMC7308994] [PubMed: 32595945](Review article on the pathogenesis of SJS/TEN and efficacy of immunosuppressive agents, focusing on possible role of intravenous immune globulin, cyclosporine and tumor necrosis factor antagonists).
- Doria A, Galecki AT, Spino C, Pop-Busui R, Cherney DZ, Lingvay I, Parsa A, et al. PERL Study Group. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med. 2020;382:2493–503. [PMC free article: PMC7375708] [PubMed: 32579810](Among 530 patients with type 1 diabetes treated with allopurinol or placebo for 3 years, decline in renal function was similar in both groups as were overall and serious adverse event rates; one patient receiving allopurinol developed a serious hepatic adverse event, but no details were provided).
- White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, Hunt B, et al. CARES Investigators. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378:1200–10. [PubMed: 29527974](Among 6190 patients with gout and cardiovascular disease treated with allopurinol or febuxostat for a median duration of 32 months, rates of overall cardiovascular events were similar in the two groups, but cardiovascular and all-cause mortality rates were higher in those on febuxostat, a surprising and unexplained outcome that was, nevertheless, supported by other studies).
- Kang DY, Yun J, Lee SY, Koh YI, Sim DW, Kim S, Nam YH, et al; Korean Severe Cutaneous Adverse Reactions Consortium. A nationwide study of severe cutaneous adverse reactions based on the multicenter registry in Korea. J Allergy Clin Immunol Pract 2020: S2213-2198(20) 30958-2. [PubMed: 32961314](Among 745 cases of severe cutaneous reactions to medication submitted to a national Korean registry between 2008 and 2016, 384 were for SJS and 361 DRESS, the most common cause of which was allopurinol [14%], carbamazepine [9.5%], vancomycin [4.7%] and antituberculosis agents [6.3%]; mortality rate was 6.6% [8.9% for SJS, 4.2% DRESS] and among 104 allopurinol implicated cases [51 SJS, 53 DRESS] the mortality was 11%).
- Mackenzie IS, Ford I, Nuki G, Hallas J, Hawkey CJ, Webster J, Ralston SH, et al. FAST Study Group. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet. 2020;396(10264):1745–57. [PubMed: 33181081](Among 6128 patients with gout and cardiovascular disease treated with either febuxostat or allopurinol for up to 7 years [mean 3.6 years], rates of combined cardiovascular endpoints were similar in the two groups [1.72 vs 2.05 events per 100 patient years] as were cardiovascular deaths [3.8% vs 4.0%]; no mention of hepatotoxicity in discussing causes of death or serious adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Delayed hypersensitivity reaction to allopurinol: A case report.[Malays Fam Physician. 2023]Delayed hypersensitivity reaction to allopurinol: A case report.Mohammad CM, Shahidah CA, Wan Fatimah SWM, Salman A, Mohd Zhafri MR, Rasimah I. Malays Fam Physician. 2023; 18:11. Epub 2023 Feb 20.
- Allopurinol and Loss of Consciousness in a 78-old Year Man Suffering from Gout.[Infect Disord Drug Targets. 2020]Allopurinol and Loss of Consciousness in a 78-old Year Man Suffering from Gout.Arian M, AkbariRad M, Moghaddam AB, Firoozi A, Jami M. Infect Disord Drug Targets. 2020; 20(2):253-256.
- Synthesis and xanthine oxidase inhibitory activity of 7-methyl-2-(phenoxymethyl)-5H-[1,3,4]thiadiazolo[3,2-a]pyrimidin-5-one derivatives.[Bioorg Med Chem. 2011]Synthesis and xanthine oxidase inhibitory activity of 7-methyl-2-(phenoxymethyl)-5H-[1,3,4]thiadiazolo[3,2-a]pyrimidin-5-one derivatives.Sathisha KR, Khanum SA, Chandra JN, Ayisha F, Balaji S, Marathe GK, Gopal S, Rangappa KS. Bioorg Med Chem. 2011 Jan 1; 19(1):211-20. Epub 2010 Nov 18.
- Review Oxipurinol: alloxanthine, Oxyprim, oxypurinol.[Drugs R D. 2004]Review Oxipurinol: alloxanthine, Oxyprim, oxypurinol.. Drugs R D. 2004; 5(3):171-5.
- Review Febuxostat: a selective xanthine-oxidase/xanthine-dehydrogenase inhibitor for the management of hyperuricemia in adults with gout.[Clin Ther. 2009]Review Febuxostat: a selective xanthine-oxidase/xanthine-dehydrogenase inhibitor for the management of hyperuricemia in adults with gout.Ernst ME, Fravel MA. Clin Ther. 2009 Nov; 31(11):2503-18.
- Allopurinol - LiverToxAllopurinol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...