NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Acitretin is a retinoid and vitamin A derivative currently used in the treatment of psoriasis. Acitretin, like many retinoids, can lead to increase in serum aminotransferase levels and has been implicated in cases of acute liver injury which can be severe and even fatal.
Background
Acitretin (a" si tre' tin) is an aromatic retinoid and the major metabolite of etretinate (e tret' i nate). Acitretin has replaced etretinate in clinical practice in the therapy of psoriasis because of its more favorable pharmacokinetics and half-life. Its mechanism of action in psoriasis is believed to be mediated by activation of retinoic acid and retinoid X receptors, which regulate gene expression important in growth and differentiation. Acitretin is considered a second generation retinoid and its relative lack of receptor specificity accounts for its many adverse side effects. All oral retinoids are potent teratogens and must be avoided or used with extreme caution in women of childbearing potential. Acitretin was approved for use in psoriasis and acne in the United States in 1996, but is currently used only in therapy of severe psoriasis unresponsive to conventional therapies and under strict regulations regarding monitoring and birth control. Acitretin is available generically and under the brand name of Soriatane in capsules of 10, 17.5, 22.5 and 25 mg, the usual dose in adults being 25 to 50 mg per day. Common side effects include dry skin, nose bleeds, conjunctivitis and hair loss. Acitretin is a known teratogen and embryotoxin. Use of acitretin has also been linked to worsening of hyperlipidemia, hyperostosis, vision and hearing loss, pancreatitis, pseudotumor cerebri, birth defects, depression and suicide.
Hepatotoxicity
Liver test abnormalities occur in up to one third of patients on acitretin, although marked elevations above three times the upper limit of normal occur in only 1% to 5%. These abnormalities are typically transient, not accompanied by symptoms and can resolve even with continuation of acitretin, but they may be associated with mild symptoms and require drug discontinuation in up to 4% of patients.
Acitretin can also cause clinically apparent liver injury with symptoms and jaundice. Although uncommon, acute liver injury from acitretin is well described and is estimated to occur in 0.1% to 0.5% of treated patients. The onset of injury can be as soon as one week or up to 9 months after starting therapy. The pattern of liver enzyme elevations is typically hepatocellular (Case 1), but cholestatic hepatitis due to acitretin has been reported (Case 2). Most cases resolve rapidly with stopping acitretin. Rash, fever, eosinophilia and other signs of hypersensitivity occur in many but not all cases; autoantibodies are rare. The injury is not at all like that of vitamin A and is not associated with fat accumulation in stellate cells. Because its potential for causing hepatotoxicity, routine monitoring of serum aminotransferase levels during acitretin therapy is recommended.
Likelihood score, acitretin: B (likely rare cause of clinically apparent liver injury).
A similar pattern of acute liver injury was reported with etretinate, a related retinoid that was previously used for psoriasis and acne, but was withdrawn from use in the United States in 1998.
Likelihood score, etretinate: B (highly likely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which acitretin causes serum aminotransferase elevation is not known. The injury does not resemble that of hypervitaminosis A and excess acitretin is not stored in the liver or in stellate cells as is vitamin A. The rare clinically apparent cases of hepatotoxicity are likely due to hypersensitivity.
Outcome and Management
The serum aminotransferase elevations that occur on acitretin therapy are usually self-limited and may not require dose modification or discontinuation of therapy. However, persistent ALT or AST elevations above 3 times the upper limit of the normal range (ULN) should lead to dose adjustment or discontinuation. Any ALT elevation above 10 times the ULN or ALT elevations with symptoms or jaundice should lead to immediate discontinuation (Case 1). The acute clinically apparent liver injury caused by acitretin is typically self-limited and benign but fatal instances (particularly with overdose or use of high doses of acitretin) have been reported. Concurrent therapy with methotrexate may increase the risk of hepatotoxicity from acitretin. No instances of chronic hepatitis or vanishing bile duct syndrome due to acitretin have been reported. Restarting acitretin after clinically apparent liver injury is usually followed by recurrence and should be avoided.
Drug Class: Dermatologic Agents; Vitamins
Other Drugs in the Subclass:
CASE REPORTS
Case 1. Mild acute hepatitis due to acitretin.(1)
A 66 year old man was found to have elevations in serum aminotransferase levels during routine monitoring 9 months after starting acitretin (50 mg daily) for pityriasis rubra psoriaform. He had no symptoms of liver disease and felt well. Past medical history included rheumatoid arthritis for which he took acetaminophen intermittently and occasional courses of prednisone, the most recent course being a few weeks before recognition of the liver injury. He used hydroxyzine regularly for itching due to his skin disease. He did not drink alcohol and had no history of liver disease, exposures to jaundiced individuals or risk factors for viral hepatitis. Physical examination was unrevealing and he had no apparent jaundice, rash, fever or signs of chronic liver disease. Laboratory testing showed marked elevations in serum ALT (1015 U/L) and AST (893 U/L), with minimal increase in alkaline phosphatase (Alk P: 212 U/L) and mild hyperbilirubinemia (2.9 mg/dL). The serum albumin (3.9 g/dL) and prothrombin time (11.2 seconds) were normal. Serum creatinine was normal, and there was no eosinophilia. Tests for hepatitis A, B and C were negative. Serum ANA and AMA were strongly positive, but he was known to have serum autoantibodies previously. Hepatic imaging was not done. Acitretin was discontinued and laboratory results improved rapidly and were normal when he was seen 8 months later (Table).
Key Points
| Medication: | Acitretin (50 mg daily) |
|---|---|
| Pattern: | Hepatocellular (R=13.2) |
| Severity: | 2+ (jaundice not requiring hospitalization) |
| Latency: | 9 months |
| Recovery: | 4 weeks |
| Other medications: | Hydroxyzine, acetaminophen |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 1 month | 0 | 37 | 54 | 0.7 | Routine monitoring |
| 5 months | 0 | 44 | 68 | 0.6 | |
| 6 months | 0 | 20 | 68 | 0.5 | |
| 7 months | 0 | 28 | 68 | 0.6 | |
| 8 months | 0 | 56 | 82 | 0.6 | |
| 9 months | 0 | 1015 | 212 | 2.9 | No symptoms |
| Acitretin discontinued because of abnormal liver tests | |||||
| 9.5 months | 2 weeks | 500 | 183 | 1.9 | |
| 10 months | 4 weeks | 41 | 99 | 0.6 | |
| 1.5 years | 9 months | 12 | 82 | 0.6 | |
| Normal Values | <45 | <125 | <1.2 | ||
Comment
The laboratory abnormalities (hepatocellular pattern of serum enzyme elevations) and clinical course (prompt resolution with stopping therapy) were compatible with acitretin induced liver injury. These abnormalities were detected during routine screening for possible liver injury in an asymptomatic patient on acitretin. The height of the elevations (>10 times ULN) and the presence of hyperbilirubinemia called for immediate discontinuation of acitretin.
Case 2. Severe acute hepatitis due to acitretin.(1)
A 58 year old man developed fatigue, anorexia, nausea and dark urine followed pruritus and jaundice 4 months after starting acitretin (50 mg daily) for psoriasis. Past medical history was otherwise unremarkable. He did not drink alcohol and had no history of liver disease or risk factors for viral hepatitis. He was also taking omeprazole which he had been taking for several years for gastroesophageal reflux. He had taken minocycline for several months but had discontinued it several weeks before becoming ill. Laboratory testing showed mild jaundice (bilirubin 4.4 mg/dL) and elevations in serum aminotransferase levels (ALT 600 U/L, AST 252 U/L) and alkaline phosphatase (Alk P: 256 U/L). The serum albumin (4.0 g/dL) and prothrombin time (INR 0.9) were normal. Acitretin was stopped promptly and he was admitted to the hospital for evaluation and monitoring (Table). Tests for hepatitis A, B, C and E were negative as were routine autoantibodies. Over the next 4 weeks his serum bilirubin continued to rise, peaking at 28.5 mg/dL. Itching was treated with cholestyramine and doxepin. Abdominal CT scans showed no evidence of biliary obstruction or hepatic masses. A liver biopsy showed severe cholestatic hepatitis suggestive of drug induced liver injury. Importantly, there was no bile duct injury or loss. He remained jaundiced for almost three months, but gradually improved and when seen in follow up 7 months after onset, he was asymptomatic and all liver tests were normal.
Key Points
| Medication: | Acitretin (50 mg daily) |
|---|---|
| Pattern: | Hepatocellular (R=7.0) initially, cholestatic (R<1) later |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 5 months |
| Recovery: | 4 months |
| Other medications: | Omeprazole |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 5 months | 0 | 600 | 256 | 4.4 | R=7.0, acitretin stopped |
| 1 day | 310 | 145 | 5.2 | R=3.8, CT scan | |
| 6 days | 286 | 317 | 8.8 | ||
| 13 days | 111 | 395 | 14.1 | R=0.9 | |
| 19 days | 62 | 425 | 20.2 | ||
| 6 months | 27 days | 59 | 438 | 24.3 | Severe pruritus |
| 33 days | 62 | 488 | 27.3 | INR 1.0 | |
| 35 days | 76 | 462 | 28.5 | Liver biopsy | |
| 41 days | 79 | 412 | 23.2 | ||
| 7 months | 62 days | 86 | 156 | 6.8 | |
| 76 days | 75 | 138 | 3.6 | ||
| 8 months | 3 months | 46 | 109 | 1.3 | |
| 1 year | 7 months | 42 | 85 | 1.2 | |
| Normal Values | <45 | <125 | <1.2 | ||
Comment
This patient suffered a somewhat severe and protracted cholestatic hepatitis which arose 5 months after starting acitretin. Despite stopping the medication, he continued to have worsening jaundice for several weeks. Laboratory, radiologic and histologic evaluations revealed no other cause of liver injury. He was treated symptomatically and did not receive corticosteroids, eventually recovering completely. The pattern of liver injury was categorized as hepatocellular with a R ratio of 7.0 initially, but serum aminotransferase levels fell promptly and alkaline phosphatase values rose so that the pattern was distinctly cholestatic after the first week. The presence of pruritus and liver histology confirmed the cholestatic nature of the hepatitis.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Acitretin – Generic, Soriatane®
DRUG CLASS
Dermatologic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
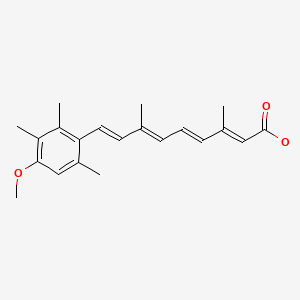
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Acitretin | 55079-83-9 | C21-H26-O3 |
|
CITED REFERENCES
- 1.
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159]
ANNOTATED BIBLIOGRAPHY
References updated: 10 November 2020
- Zimmerman HJ. Vitamin A(retinol). Drugs used in dermatotherapy. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 727-9.(Expert review of hepatotoxicity of vitamin A and the retinoids published in 1999; mentions two published reports of acute necrosis due to acitretin).
- Liu LU, Schiano TD. Vitamin A (retinol). Hepatotoxicity of herbal medications, vitamins and natural hepatotoxins. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 744-6.(Review of vitamin A and retinoid hepatotoxicity published in 2007).
- Sewell MJ, Burkhart C, Morrell D. Dermatological pharmacology. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1271-96.(Textbook of pharmacology and therapeutics).
- Peck GL, Olsen TG, Yoder FW, Strauss JS, Downing DT, Pandya M, Butkus D, et al. Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid. N Engl J Med. 1979;300:329–33. [PubMed: 153472](Initial report on use of isotretinoin for severe acne mentions that mild and transient ALT elevations occurred in one of the 14 patients treated for 4 months).
- Thune P, Mork NJ. A case of centrolobular toxic necrosis of the liver due to aromatic retinoid--Tigason (Ro-10-9359). Dermatologica. 1980;160:405–8. [PubMed: 7389973](54 year old woman with ichthyosis developed fatigue 2-3 months after starting etretinate [peak bilirubin 2.6 mg/dL, ALT 1260 U/L, Alk P 350 U/L], biopsy showing centrolobular necrosis, with slow resolution over the next 6 months).
- Peck GL, Olsen TG, Butkus D, Pandya M, Arnaud-Battandier J, Gross EG, Windhorst DB, et al. Isotretinoin versus placebo in the treatment of cystic acne. A randomized double-blind study. J Am Acad Dermatol. 1982;6 Suppl:735–45. [PubMed: 6461677](Controlled trial of 4 months of isotretinoin in 33 patients with severe acne showed dramatic therapeutic effect; side effects were dry skin, eyes and nasal membranes and dermatitis; ALT elevations occurred in 3 patients [10%], but levels returned to normal despite continuing and no patient stopped therapy because of side effects).
- Glazer SD, Roenigk HH Jr, Yokoo H, Sparberg M. A study of potential hepatotoxicity of etretinate used in the treatment of psoriasis. J Am Acad Dermatol. 1982;6 Suppl:683–7. [PubMed: 7068976](20 patients with psoriasis underwent liver biopsy before and/or up to 6 months after starting etretinate, 20% had ALT elevations, but no worsening of fibrosis or consistent effect on histology).
- Olson JA. Adverse effects of large doses of vitamin A and retinoids. Semin Oncol. 1983;10:290–3. [PubMed: 6364354](Review of retinoids and vitamin A which do not share same toxicity, but usually have a low therapeutic-toxic index).
- Beck HI, Foged EK. Toxic hepatitis due to combination therapy with methotrexate and etretinate in psoriasis. Dermatologica. 1983;167:94–6. [PubMed: 6628806](47 year old woman with psoriasis who had been on oral weekly methotrexate for 10 years with normal liver tests, developed fever, jaundice and ascites 4 months after adding 25-75 mg/day etretinate [bilirubin 17 mg/dL, AST 460 U/L, Alk P 1.5 times ULN, prothrombin index 27%], with resolution 2 months after stopping both agents; liver biopsy later showed cirrhosis).
- van Voorst Vader PC, Houthoff HJ, Eggink HF, Gips CH. Etretinate (Tigason) hepatitis in 2 patients. Dermatologica. 1984;168:41–6. [PubMed: 6698264](2 women with etretinate hepatotoxicity; 70 and 61 year olds with onset of liver tests abnormalities 6 and 3 months after starting etretinate [bilirubin normal, ALT 31 and 476 U/L, Alk P 166 and 147 U/L], resolving within 2-3 months of stopping and recurring within a month of rechallenge or switching to a lower dose [while ALT remaining mildly abnormal]).
- Danan G, Pessayre D, Rueff B, Benhamou JP. Gastroenterol Clin Biol. 1984;8:770–1. [Acute hepatitis probably due to Plethoryl] French. [PubMed: 6549301](3 women, ages 25 to 44 years, developed symptoms of hepatitis 2-10 weeks after starting Plethoryl, a mixture including 50,000 IU of a retinoid marketed in Europe for treatment of obesity [bilirubin 2.6-3.8 mg/dL, ALT 490-576 U/L, Alk P normal], resolving within 4-8 weeks of stopping).
- Foged E, Bjerring P, Kragballe K, Sød H, Zachariae H. Histologic changes in the liver during etretinate treatment. J Am Acad Dermatol. 1984;11(4 Pt 1):580–3. [PubMed: 6490982](Among 32 patients treated with etretinate for 6-60 months undergoing liver biopsy, changes included mild fatty change, nuclear variability and minimal fibrosis, but similar changes were found in controls).
- Weiss VC, West DP, Ackerman R, Robinson LA. Hepatotoxic reactions in a patient treated with etretinate. Arch Dermatol. 1984;120:104–6. [PubMed: 6691707](74 year old woman developed fatigue and fever 1 month after starting etretinate [bilirubin normal, ALT 545 U/L, Alk P 265 U/L, eosinophils 14-24%], rapidly improving upon stopping and recurring within one month of restarting).
- Gavish D, Katz M, Gottehrer N, Israeli A, Lijovetzky G, Holubar K. Cholestatic jaundice, an unusual side effect of etretinate. J Am Acad Dermatol. 1985;13:669–70. [PubMed: 4078057](58 year old man with psoriasis developed jaundice and pruritus 12 weeks after starting etretinate [bilirubin rising to 23.4 mg/dL, ALT 60 U/L, Alk P 420 U/L], resolving within 4 weeks of stopping; biopsy showed cholestatic hepatitis).
- Grimaud JC, Langier R, Costa-Legre MC, Payan MJ. Presse Med. 1985;14:844–5. [Hepatitis due to etretinate] French. [PubMed: 3158914](62 year old man with psoriasis developed abnormal serum enzyme levels 10 days after restarting etretinate which had been given for 3 months in the past [ALT 420 U/L, Alk P 176 U/L, bilirubin not given], resolving within 2 months of stopping).
- Weiss VC, Layden T, Spinowitz A, Buys CM, Nemchausky BA, West DP, Emmons KM. Chronic active hepatitis associated with etretinate therapy. Br J Dermatol. 1985;112:591–7. [PubMed: 4005158](39 year old man with psoriasis developed nausea 6 months after starting etretinate [bilirubin normal, ALT 495 U/L, Alk P 141 U/L], with persistence of ALT elevations for 6 months after stopping and biopsy showing "chronic active hepatitis").
- Heller EH, Shiffman NJ. Synthetic retinoids in dermatology. Can Med Assoc J. 1985;132:1129–36. [PMC free article: PMC1345937] [PubMed: 3158386](Discussion of two synthetic analogues of vitamin A introduced into dermatology: isotretinoin [used in acne] and etretinate [used in psoriasis]; these retinoids are less toxic than vitamin A and not stored in the liver, although they are metabolized there; minor liver test abnormalities may arise during therapy, but rarely required dose modification).
- Vahlquist A. LöL, Nordlinder H, Rollman O, Vahlquist C. Differential hepatotoxicity of two oral retinoids (etretinate and isotretinoin) in a patient with palmoplantar psoriasis. Acta Derm Venereol. 1985;65:359–62. [PubMed: 2413699](64 year old woman was found to have abnormal liver tests 5 months after starting etretinate [bilirubin 0.7 mg/dL, ALT 950 U/L, Alk P 402 U/L], liver biopsy showing hepatitis without steatosis, resolving with stopping and prednisone therapy, recurring upon rechallenge, but not when isotretinoin was started which was less effective for the psoriasis).
- Atttali P, Bernuau J, Fabre M, Degott C, Ink O. Gastroenterol Clin Biol. 1986;10:92. [Fatal fulminant hepatitis probably due to Plethoryl] French. [PubMed: 3754231](22 year old developed fatigue 2 weeks and jaundice 3 weeks after starting Plethoryl, a combination drug for obesity which includes a retinoid [bilirubin 12.3 mg/dL, ALT 5,500 U/L], progressing to hepatic failure and death one month later).
- Jean-Pastor MJ, Jean P, Biour M, Castot A, Chichmanian F, Danan G, Levy VG, et al. J Toxicol Clin Exp. 1986;6:115–21. [Hepatopathies from treatment with a specialty drug combination of tiratricol-cyclovalone-retinol] French. [PubMed: 3783484](Summary of 9 cases of liver injury attributed to use of Plethoryl, 6 of which were considered convincing; all in obese women, ages 24 to 50 years with latency to onset of 2 weeks to 2 years, 3 with jaundice, all resolving after stopping and 3 recurring upon reexposure, injury attributed to a retinol [50,000 IU] in the commercial product).
- Marti R, Voiment YM, André M. Gastroenterol Clin Biol. 1986;10:853. [Acute hepatitis caused by Plethoryl] French. [PubMed: 3803829](36 year old woman developed fever and arthralgias 3 months after starting Plethoryl, a drug combination for obesity which includes a retinoid [bilirubin 4.0 mg/dL, ALT 708 U/L, Alk P 137 U/L], resolving rapidly upon stopping and recurring within 5 days of restarting).
- Camuto P, Shupack J, Orbuch P, Tobias H, Sidhu G, Feiner H. Long-term effects of etretinate on the liver in psoriasis. Am J Surg Pathol. 1987;11:30–7. [PubMed: 3789256](18 patients treated with etretinate for psoriasis for at least 5 years underwent liver biopsy; 2 had persistent liver test elevations, one had Alk P >500 U/L with a "normal" liver biopsy, one had ALT of 125 U/L with chronic hepatitis and fibrosis on biopsy [no HCV testing available], a third had normal tests but had cirrhosis on biopsy; many biopsies showed fatty change and occasional stellate cell hypertrophy).
- Yob EH, Pochi PE. Side effects and long-term toxicity of synthetic retinoids. Arch Dermatol. 1987;123:1375–8. [PubMed: 3310911](Retinoids are modifications of vitamin A molecule and are not stored in the liver; hepatotoxicity of retinoids is different from that of vitamin A, usually arising within first 1-2 months and having features of hypersensitivity).
- Khouri MR, Saul SH, Dlugoz AA, Soloway RD. Hepatocanalicular injury associated with vitamin A derivative etretinate. Dig Dis Sci. 1987;32:1207–11. [PubMed: 3652901](73 year old man with psoriasis developed fever 3-4 weeks after starting etretinate [bilirubin 0.8 mg/dL, ALT 126 U/L, Alk P 383 U/L, eosinophils 1368/μL], jaundice arising with continuation of drug for 5 days [peak bilirubin 2.5 mg/dL], resolving within 2 weeks of stopping).
- Kingston TP, Matt LH, Lowe NJ. Etretin therapy for severe psoriasis. Arch Dermatol. 1987;123:55–8. [PubMed: 2948451](Open label study of etretin [acitretin] in 21 patients with psoriasis, 3 required dose modification for liver test elevations, but few details given).
- Larrey D, Fréaux E, Babany G, Berson A, Amée-Manesme O, Degott C, Bettan L, et al. Gastroenterol Clin Biol. 1988;12:240–4. [Hepatitis probably caused by Plethoryl. Apropos of 7 cases] French. [PubMed: 3371597](Seven cases of liver injury in women taking Plethoryl [a retinoid containing combination used to treat obesity], arising after 3-16 weeks of therapy [bilirubin 3.3-26.3 mg/dL, ALT 21-61 times ULN, Alk P 0.9-1.8 times ULN], resolving within 2-3 months of stopping).
- Prudent A, Marchetti B, Philibert P, Lacroix G. Legré. Ann Gastroenterol Hepatol (Paris). 1988;24:27. [Acute recurrent hepatitis caused by plethoryl] French. [PubMed: 3355100](48 year old woman developed nausea, fatigue, and arthralgias 6 month after starting Plethoryl for weight loss [bilirubin 2.2 mg/dL, ALT 588 U/L, Alk P 448 U/L], which resolved on stopping and recurred within a week of restarting on two occasions).
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273–88. [PubMed: 3054426](Review of pharmacology, clinical effectiveness and low dose, long term toxicities of the retinoids; states that therapy usually has little effect on ALT or bilirubin levels).
- Roenigk HH Jr. Liver toxicity of retinoid therapy. J Am Acad Dermatol. 1988;19(1 Pt 2):199–208. [PubMed: 3045164](Review of hepatotoxicity of vitamin A and the retinoids).
- Thirumoorthy T, Shupack JL. Adverse hepatic reactions associated with etretinate in patients with psoriasis--analysis of 22 cases. Ann Acad Med Singapore. 1988;17:477–81. [PubMed: 3066276](Among 533 patients with psoriasis treated with etretinate, there were 22 reports of hepatic adverse events [4%]; 6 were considered probably related, 7 possibly, 6 unlikely and 3 unclassifiable; 2 patients developed fever, malaise and rash 4-5 weeks after starting etretinate with rapid rise in ALT and rapid resolution on stopping; others had serum enzyme elevations without symptoms or jaundice).
- Zachariae H. Dangers of methotrexate/etretinate combination therapy. Lancet. 1988;1(8582):422. [PubMed: 2893228](2 of 10 patients on the combination of etretinate and methotrexate developed severe liver injury, including a 47 year old man who developed jaundice 1 month after adding etretinate to chronic methotrexate therapy [bilirubin 17.1 mg/dL, AST 305 U/L, Alk P 742 U/L], which resolved within 2 months of stopping both).
- Causse X, Paliard P. Gastroenterol Clin Biol. 1989;13:526–7. [Hepatitis with auto-immunization probably caused by Plethoryl] French. [PubMed: 2753297](32 year old woman developed jaundice 4-6 weeks after starting Plethoryl for obesity [bilirubin 12.2 mg/dL, ALT 1690 U/L], but continued drug for 2 months and developed ANA 1:1024, responding to course of corticosteroid therapy; ultimately liver tests were normal and ANA fell to 1:40; Plethoryl [a drug combination that included a synthetic retinoid] was withdrawn from market in France in 1988).
- Gupta AK, Goldfarb MT, Ellis CN, Voorhees JJ. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088–93. [PubMed: 2526824](Controlled trial of 4 different doses of acitretin in 38 patients with psoriasis, followed by open label long term use, 15% had ALT or AST elevations during therapy, rising above 100 U/L in two patients, without jaundice, and resolving rapidly on stopping).
- Maroy B, Moullot P, Constantin JM. Presse Med. 1989;18:567–70. [Probable side effects caused by plethoryl. Common acute hepatitis, anicteric hepatitis, cirrhosis due to hypervitaminosis A, inflammatory arthralgias] French. [PubMed: 2523055](4 cases of adverse events due to use of Plethoryl, including 2 instances of acute hepatocellular injury, 1 of cirrhosis and 1 of arthritis which improved on stopping Plethoryl).
- Roenigk HH Jr. Liver toxicity of retinoid therapy. Pharmacol Ther. 1989;40:145–55. [PubMed: 2645587](Review of toxicity of retinoids including description of liver biopsy results described by Glazer [1982]).
- Kragballe K, Jansen CT, Geiger JM, Bjerke JR, Falk ES, Gip L, Hjorth N, et al. A double-blind comparison of acitretin and etretinate in the treatment of severe psoriasis. Results of a Nordic multicentre study. Acta Derm Venereol. 1989;69:35–40. [PubMed: 2563606](Controlled trial of acitretin [n=127] vs etretinate [n=41] in psoriasis found similar efficacy; AST elevations occurred in 12% vs 11% and one patient on acitretin for 12 weeks developed biopsy proven "toxic hepatitis" which resolved on stopping).
- Olsen EA, Weed WW, Meyer CJ, Cobo LM. A double-blind, placebo-controlled trial of acitretin for the treatment of psoriasis. J Am Acad Dermatol. 1989;21:681–6. [PubMed: 2530251](Controlled trial of acitretin in 15 patients with psoriasis, including a 28 year old man who developed fatigue and elevated liver tests [bilirubin 0.5 mg/dL, ALT 396 U/L, Alk P 140 U/L], which resolved within 2 months of stopping).
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, van de Kerkhof PC, Yap SH. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185–8. [PubMed: 2147707](50 year old woman with psoriasis developed elevated liver tests 4-5 months after starting acitretin [bilirubin normal, ALT 790 U/L, Alk P 166 U/L] and worsened for 2 months after stopping [bilirubin rising to 3.8 mg/dL]; liver biopsy showed severe hepatitis with bridging necrosis and, on follow up, an inactive cirrhosis despite all liver tests returning to normal).
- Fallon MB, Boyer JL. Hepatic toxicity of vitamin A and synthetic retinoids. J Gastroenterol Hepatol. 1990;5:334–42. [PubMed: 2103414](Review of liver injury due to hypervitaminosis A and retinoids identified 18 reports of vitamin A hepatotoxicity in the English literature, patient ages 6 to 63 years, presenting with rash, fatigue, hepatomegaly and hepatic synthetic dysfunction, biopsy showing fat in stellate cells and fibrosis; little evidence that isotretinoin causes liver injury other than mild rapidly reversible ALT elevations; etretinate causes ALT elevations in ~20% of patients and case reports of clinically apparent injury have been published, but vary in clinical patterns).
- Marhold I, Duschet P, Schwarz T, Gschnait F. Hautarzt. 1991;42:580–3. [Successful use of isotretinoin in type Zumbusch generalized pustular psoriasis following recovered etretinate-induced hepatitis] German. [PubMed: 1938411](29 year old woman with psoriasis responded to etretinate but developed rising Alk P levels [172 to 1736 U/L] without jaundice and only mild ALT increases [38 to 178 U/L] 24 days after starting therapy, which improved on stopping and she later tolerated isotretinoin without recurrence [Alk P 118 U/L, ALT 14 U/L]).
- Dubois A, Balducchi JP, Barbuat C, Fabre J, Flaisler F, Joujoux JM, Pignodel C, et al. Rev Med Interne. 1991;12:295–8. [Portal hypertension and hypervitaminosis A. Apropos of 2 cases and review of the literature] French. [PubMed: 1759070](Two patients presented with ascites and biopsy showing stellate cell hypertrophy one having taken methoxypsoralen [for tanning] and one Plethoryl [for weight loss] for several years, improving on stopping).
- Green C, Lakshmipathi T. A case of hepatitis related to etretinate therapy and hepatitis B vaccine. Dermatologica. 1991;182:119–20. [PubMed: 1828773](41 year old man with psoriasis had mild fluctuations in serum AST during etretinate therapy ultimately [at 9 months] rising to 361 U/L, without jaundice or symptoms and resolving rapidly with discontinuation).
- Murray HE, Anhalt AW, Lessard R, Schacter RK, Ross JB, Stewart WD, Geiger JM. A 12-month treatment of severe psoriasis with acitretin: results of a Canadian open multicenter study. J Am Acad Dermatol. 1991;24:598–602. [PubMed: 1827800](Open label study of 12 month course of acitretin in 63 patients with psoriasis, ALT elevations occurred in ~10% of patients, one requiring discontinuation [ALT 3 times ULN at 5 months, resolving within two months of stopping]).
- Vahlquist A. Long-term safety of retinoid therapy. J Am Acad Dermatol. 1992;27(6 Pt 2):S29–33. [PubMed: 1460122](Patients have been treated with retinoids for up to 15 years, generally without toxicity; retinoids do not accumulate in the liver and do not cause accumulation of fat droplets in stellate cells as occurs with hypervitaminosis A; two types of hepatotoxicity, one idiosyncratic acute hepatitis typically occurring with aromatic retinoids and one a long term low grade injury that can lead to cirrhosis, perhaps aggravated by alcohol that the author claims can occur with all retinoids).
- Mørk NJ. Kolbenstvedt, Austad J. Efficacy and skeletal side effects of two years' acitretin treatment. Acta Derm Venereol. 1992;72:445–8. [PubMed: 1362840](One of 51 patients with psoriasis treated with acitretin for up to 2 years developed "toxic hepatitis" which arose after 5 months of treatment and resolved 20 weeks after stopping; few details given).
- Pilkington T, Brodgen RN. Acitretin: a review of its pharmacology and therapeutic use. Drugs. 1992;43:597–627. [PubMed: 1377120](Extensive review of the pharmacology, clinical efficacy, and toxicity of acitretin; vitamin A-like side effects are common such as dry skin, lips, eyes and nose, skin desquamation, alopecia, fatigue and pruritus; ALT elevations occur in 16%, but are usually asymptomatic, although cases requiring drug withdrawal have been reported).
- Coschieri M, Philippon A, Quinsat D, Dor JF, Chichmanian RM. Acute hepatitis involvement during ingestion of acitretin. Gastroenterol Clin Biol. 1993;17:769–70. [PubMed: 8288093](80 year old man developed nausea and rash one week after starting acitretin [bilirubin normal, ALT 21 times ULN, Alk P normal], resolving within a month of stopping).
- Sanchez MR, Ross B, Rotterdam H, Salik J, Brodie R, Freedberg IM. Retinoid hepatitis. J Am Acad Dermatol. 1993;28(5 Pt 2):853–8. [PubMed: 8491880](65 year old woman developed elevated ALT levels 2 months after starting etretinate [bilirubin 2.9 rising to 6.7 mg/dL, ALT 820 U/L, Alk P 250 U/L], worsening for 2 months after stopping and then resolving).
- Kano Y, Fukuda M, Shiohara T, Nagashima M. Cholestatic hepatitis occurring shortly after etretinate therapy. J Am Acad Dermatol. 1994;31:133–4. [PubMed: 8021361](65 year old man with psoriasis developed fever and jaundice within 7 days of starting etretinate, resolving within 2 weeks of stopping; few details given).
- Shibata K, Shimamoto Y, Ishibashi S, Tominaga H, Suga K, Yamaguchi M. Life-threatening hepatic toxicity caused by all-trans-retinoic acid in a patient with acute promyelocytic leukaemia. Clin Lab Haematol. 1994;16:191–5. [PubMed: 7955929](39 year old man with acute promyelocytic leukemia developed jaundice one month after starting all-trans-retinoic acid with peak bilirubin ~10 mg/dL, resolving in 18 days after stopping and patient achieving remission despite early discontinuation).
- Krüger-Krasagakes S, Grabbe J, Czarnetzki BM. Possible aggravation of hepatitis A by acitretin. Acta Derm Venereol. 1995;75:82–3. [PubMed: 7747547](49 year old man developed fatigue 1 month and fever and jaundice 2 months after starting acitretin [bilirubin ~12 mg/dL, ALT ~2500 U/L, Alk P normal, protime 50%], but was also IgM anti-HAV positive; authors hypothesize that acitretin like vitamin A may worsen the course of acute viral hepatitis).
- Stern RS, Fitzgerald E, Ellis CN, Lowe N, Goldfarb MT, Baughman RD. The safety of etretinate as long-term therapy for psoriasis: results of the etretinate follow-up study. J Am Acad Dermatol. 1995;33:44–52. [PubMed: 7601945](Analysis of prospective survey of 956 patients with psoriasis treated with etretinate for up to 5 years; 66 patients reported hepatic problems during study including 13 with hepatitis and 13 with cirrhosis [5 with both], but relationship to therapy could not be assessed).
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 Pt 2):S7–S12. [PubMed: 10459140](Review of side effects of acitretin based upon data from 1877 patients reported "overt chemical hepatitis" in 0.26%; among 128 patients undergoing routine pre- and post-treatment liver biopsies, 83% were improved or unchanged; authors recommend routine monitoring of liver tests every 1-2 weeks "until stable, and thereafter at intervals as clinically indicated").
- Roenigk HH Jr, Callen JP, Guzzo CA, Katz HI, Lowe N, Madison K, Nigra T, et al. Effects of acitretin on the liver. J Am Acad Dermatol. 1999;41:584–8. [PubMed: 10495381](Among 128 patients treated with acitretin, 83 underwent liver biopsy before and 2 years after starting therapy; 30% had ALT elevations, liver histology did not change on average; one patient developed fibrosis, but had no serum enzyme worsening).
- Perea G, Salar A, Alté A, Brunet S, Sierra J. Acute hepatomegaly with severe liver toxicity due to all-trans-retinoic acid. Haematologica. 2000;85:551–2. [PubMed: 10800178](40 year old man with promyelocytic leukemia developed jaundice 21 days after starting all-trans-retinoic acid and idarubicin [direct bilirubin 2.3 mg/dL, ALT normal, Alk P 370 U/L], biopsy showing intrahepatic cholestasis and resolving within 2 weeks of stopping).
- Mawson AR, Steele TA. Possible role of retinoids in hepatitis B virus-associated liver damage. Exp Biol Med (Maywood). 2001;226:734–9. [PubMed: 11520938](Review and hypothesis regarding interactions of vitamin A, retinoids and hepatitis B virus infection).
- Kreiss C, Amin S, Nalesnik MA, Chopra K, Shakil AO. Severe cholestatic hepatitis in a patient taking acitretin. Am J Gastroenterol. 2002;97:775–7. [PubMed: 11922592](51 year old man with psoriasis developed jaundice 3 months after starting acitretin [bilirubin ~12 rising to 70 mg/dL, initial ALT ~4500 U/L, Alk P ~550 U/L] and had a prolonged relapsing course despite prednisone therapy; liver biopsy showed intrahepatic cholestasis and fibrosis).
- Van Zander J, Orlow SJ. Efficacy and safety of oral retinoids in psoriasis. Expert Opin Drug Saf. 2005;4:129–38. [PubMed: 15709903](Review of acitretin and other retinoids as therapy of psoriasis; side effects include ALT elevations in one third of treated patients, but clinically apparent liver injury is rare).
- Pang ML, Murase JE, Koo J. An updated review of acitretin-a systemic retinoid for the treatment of psoriasis. Expert Opin Drug Metab Toxicol. 2008;4:953–64. [PubMed: 18624682](Side effects of acitretin are somewhat dose related, liver enzyme elevations in 25-30% of patients, but usually with high dose therapy, occurring 2-8 weeks after starting therapy and transient or responding to dose modification).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, one was attributed to acitretin: Case 1 for acitretin).
- Leithead JA, Simpson KJ, MacGilchrist AJ. Fulminant hepatic failure following overdose of the vitamin A metabolite acitretin. Eur J Gastroenterol Hepatol. 2009;21:230–2. [PubMed: 19092674](42 year old woman took an 600 mg overdose of acitretin and presented 2 days later with acute liver failure [bilirubin 5.7 mg/dL, ALT 10,226 U/L, Alk P 153 U/L, protime 51 seconds and renal dysfunction]; acitretin levels were undetectable and she recovered spontaneously).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](Among 313 cases of drug induced liver injury seen between 1997 and 2008 at a large hospital in Bangalore, India, no cases were attributed to vitamin A or retinoids).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to vitamin A or a retinoid).
- Mas Morey P, Nigorra Caro M, Cladera Serra A, Nicolás Picó J. Possible fulminant toxicity by all-trans-retinoic acid in a patient with acute promyelocytic leukemia. Farm Hosp. 2011;35:44–5. [PubMed: 20605103](33 year old woman with promyelocytic leukemia treated with all-trans-retinoic acid developed acute liver failure within 3 weeks of starting [bilirubin 1.1 rising to 17.4 mg/dL], with progressive pulmonary and renal failure and death 25 days after starting).
- Stickel F, Kessebohm K, Weimann R, Seitz HK. Review of liver injury associated with dietary supplements. Liver Int. 2011;31:595–605. [PubMed: 21457433](Review of the hepatotoxicity of herbals and nutritional supplements including vitamin A, toxic levels being above 50,000 IU daily, but toxic dose is lower in persons with risk factors such as underlying liver disease).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, three of which were attributed to isotretinoin [ranking 6th in frequency] all of which were anicteric and only one symptomatic).
- Lérisson M, Ripault MP, Pageaux GP, Guillot B, Larrey D. Hepatitis after retinoid percutaneous administration. Clin Res Hepatol Gastroenterol. 2014;38:e99–e101. [PubMed: 24969684](55 year old woman with Darier disease developed anicteric hepatitis 10 months after starting acitretin [bilirubin 0.4 mg/dL, 107 U/L, Alk P 319 U/L] which resolved on stopping, but reappeared 8 months after starting topical acitretin [bilirubin 0.7 mg/dL, ALT 403 U/L, Alk P 203 U/L], resolving 6 months after stopping).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, two of which were attributed to retinoids).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 3 cases were attributed to acitretin, but none to other retinoids or vitamin A).
- Homma Y, Otani N, Ishimatsu S. A case report of acute vitamin A intoxication due to ocean perch liver ingestion. J Emerg Med. 2015;49:15–7. [PubMed: 25850632](27 year old man developed flushing, headache, nausea and joint pains one day after eating 800 g of ocean perch liver, with normal liver enzymes, but high retinol levels and subsequent facial desquamation).
- Mengual-Moreno E, Lizarzábal-García M, Ruiz-Soler M, Silva-Suarez N, Andrade-Bellido R, Lucena-González M, Bessone F, et al. Invest Clin. 2015;56:3–12. [Case reports of drug-induced liver injury in a reference hospital of Zulia state, Venezuela] Spanish. [PubMed: 25920181](During a one year period, 13 cases of drug induced liver injury were seen at a single hospital in Venezuela, the implicated agents being acetaminophen [3], ibuprofen [3], isoniazid [2], Herbalife products [2: one fatal], and 1 each of isotretinoin, amoxicillin/clavulanate and methotrexate).
- Sauder MB, Cheung L, Beecker J. Acitretin-induced hepatitis: when to monitor cholestatic enzymes. J Cutan Med Surg. 2015;19:115–20. [PubMed: 25775629](69 year old woman with bullous lichen sclerosus developed serum enzyme elevations without symptoms or jaundice within 2 weeks of starting acitretin and prednisone [bilirubin normal, peak ALT 328 U/L, Alk P 295 U/L], improving rapidly upon stopping acitretin, later tolerating methotrexate).
- Borghi A, Corazza M, Bertoldi AM, Caroppo F, Virgili A. Low-dose acitretin in treatment of plaque-type psoriasis: descriptive study of efficacy and safety. Acta Derm Venereol. 2015;95:332–6. [PubMed: 24978351](Among 46 patients with moderate-to-severe plaque psoriasis treated with acitretin, the response rate was 67% and adverse events occurred in 39%, which was mostly alopecia, dry eyes, cheilitis, fatigue, hyperlipidemia and gastrointestinal upset, and only one person [2%] was listed as having elevated aminotransferase levels).
- Drugs for psoriasis. Med Lett Drugs Ther. 2019;61(1574):89–96. [PubMed: 31381544](Concise review of the drugs approved for use in psoriasis including their mechanism of action, clinical efficacy, safety and costs, mentions that acitretin has significant mucocutaneous toxicity with cheilitis, hair loss, dry skin and desquamation and that about one third of patients have ALT elevations during therapy that are usually mild and transient but can evolve into clinically significant liver injury and can lead to cirrhosis).
- Nikolaou V, Patsatsi A, Sidiropoulou P, Chlouverakis G, Kavvalou E, Koletsa T, Economidi A, et al. Monotherapy and combination therapy with acitretin for mycosis fungoides: results of a retrospective, multicentre study. J Eur Acad Dermatol Venereol. 2020 May 4; Epub ahead of print. [PubMed: 32364303](Among 128 patients with mycosis fungoides treated with acitretin starting with low doses, the overall response rate was 77% and the adverse event rate 48%, most commonly dyslipidemia [24%], xerosis [19%] and hair loss [8%]; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Retinoids.[LiverTox: Clinical and Researc...]Review Retinoids.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Isotretinoin.[LiverTox: Clinical and Researc...]Review Isotretinoin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Retinoids and psoriasis: novel issues in retinoid pharmacology and implications for psoriasis treatment.[J Am Acad Dermatol. 1999]Review Retinoids and psoriasis: novel issues in retinoid pharmacology and implications for psoriasis treatment.Saurat JH. J Am Acad Dermatol. 1999 Sep; 41(3 Pt 2):S2-6.
- Review A review of acitretin, a systemic retinoid for the treatment of psoriasis.[Expert Opin Pharmacother. 2005]Review A review of acitretin, a systemic retinoid for the treatment of psoriasis.Lee CS, Koo J. Expert Opin Pharmacother. 2005 Aug; 6(10):1725-34.
- The frequent use of oral retinoids in combination with other treatments for psoriasis: a retrospective analysis.[J Cutan Med Surg. 2004]The frequent use of oral retinoids in combination with other treatments for psoriasis: a retrospective analysis.Hu J, Balkrishnan R, Camacho F, Lang W, Pearce DJ, Fleischer AB Jr, Feldman SR. J Cutan Med Surg. 2004 Nov-Dec; 8(6):411-4.
- Acitretin - LiverToxAcitretin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...