NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Bexarotene is a retinoid analogue that is used to treat the skin manifestations of cutaneous T cell lymphoma (CTCL). Bexarotene therapy is associated with a high rate of serum enzyme elevations and rare instances of clinically apparent acute liver injury.
Background
Bexarotene (bex ar' oh teen) is a synthetic retinoid analogue and antineoplastic agent that acts through engagement of the retinoid receptors to regulate genes involved in cellular differentiation and growth. Bexarotene is considered a third generation retinoid because it has vitamin A-like activities, but is designed to optimize binding to retinoid X receptors (RXRs) which typically lead to malignant cell apoptosis as opposed to retinoic acid receptors (RARs) which affect cellular differentiation and cell growth. Bexarotene has potent activity on tumor cell lines and has been shown to induce partial or complete remissions in up to half of patients with cutaneous T cell lymphoma (CTCL) refractory to standard therapy. Bexarotene was approved for use in CTCL in 2000 and remains a second line therapy for the cutaneous manifestations of this form of lymphoma. Bexarotene is available as capsules of 75 mg under the brand name Targretin. The recommended dosage is 300 mg/m2 daily. Common side effects include hyperlipidemia, headache, weakness, leukopenia, anemia, infection, dry skin, rash and photosensitivity. Rare, but severe adverse events include cataracts, neutropenia, hypothyroidism, pancreatitis and hepatitis. Bexarotene, like other retinoids, is teratogenic and contraindicated during pregnancy and in women who are unable to practice adequate methods of birth control.
Hepatotoxicity
Serum aminotransferase elevations occur in 5% of patients treated with bexarotene, but the abnormalities are generally mild, transient and not associated with symptoms or jaundice. However, cases of clinically apparent liver injury with jaundice have been reported with bexarotene therapy, some of which were severe and even fatal. Hepatotoxicity appears to be more common with higher doses. The clinical features of the hepatic injury with bexarotene have not been described in any detail and case reports of hepatotoxicity have yet to be published. Nevertheless, the product label mentions hepatotoxicity and recommends prospective monitoring of routine liver tests.
Likelihood score: D (possible but uncommon cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the liver injury from bexarotene and other retinoids is unknown, but may relate to their effects in inducing apoptosis. The clinically apparent liver injury from bexarotene is said to have features of hypersensitivity. Bexarotene is extensively metabolized by CYP 3A4 in the liver and is susceptible to multiple drug-drug interactions. Inhibitors of CYP 3A4 such as ketoconazole and clarithromycin can cause increases in bexarotene plasma levels with subsequent toxicity and, therefore, should be avoided.
Outcome and Management
The severity of the liver injury linked to bexarotene therapy has ranged from mild, transient and asymptomatic serum enzyme elevations to clinically apparent cholestasis to acute liver failure. Bexarotene has not been linked to cases of chronic hepatitis or vanishing bile duct syndrome. There is no information on cross sensitivity to hepatic injury between bexarotene and other retinoids, but some degree of cross sensitivity is likely.
Drug Class: Antineoplastic Agents, Retinoids
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bexarotene – Targretin®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Bexarotene | 153559-49-0 | C24-H28-O2 |
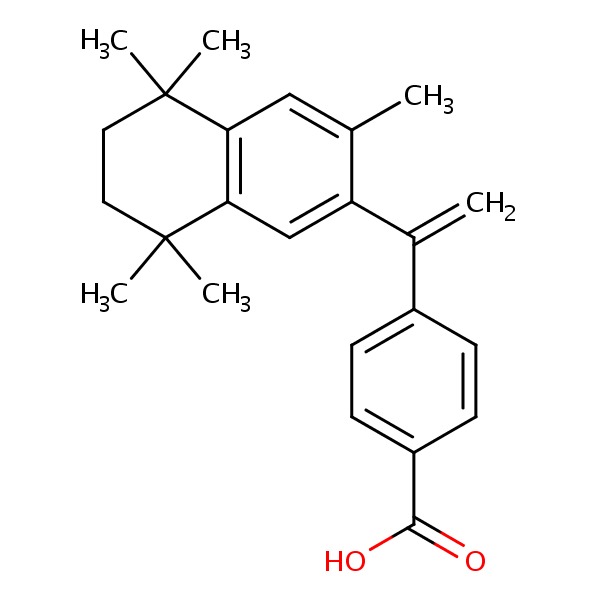 |
| Vitamin A | 68-26-8 | C20-H30-O |
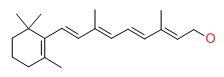 |
ANNOTATED BIBLIOGRAPHY
References updated: 26 September 2017
Abbreviations: CTCL, cutaneous T cell lymphoma
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; discusses liver injury from retinoids, but not specifically bexarotene).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 541-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; bexarotene is not discussed).
- Burkhart C, Morrell D, Goldsmith L. Dermatological pharmacology. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp 1803-32.(Textbook of pharmacology and therapeutics).
- Bexarotene (Targretin) for cutaneous T-cell lymphoma. Med Lett Drugs Ther 2000; 42 (1075): 31-2. [PubMed: 10788961](Concise review of the mechanism of action, clinical efficacy, adverse effects and costs of bexarotene shortly after its approval in the US; mentions that “elevations in aminotransferase concentrations have occurred, and fatal pancreatitis and fatal cholestasis have been reported”).
- Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, Crowley C, et al.; Bexarotene Worldwide Study Group. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol 2001; 19: 2456-71. [PubMed: 11331325](Among 94 patients with advanced and refractory CTCL treated with bexarotene, clinical responses occurred in 45-55% and side effects were common but usually mild, but led to early termination of therapy in 10%; AST elevations occurred during the first month of treatment in 20% of patients).
- Duvic M, Martin AG, Kim Y, Olsen E, Wood GS, Crowley CA, Yocum RC; Worldwide Bexarotene Study Group. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol 2001; 137: 581-93. [PubMed: 11346336](Among 58 patients with refractory or persistent CTCL treated with bexarotene at different doses, clinical responses occurred in 56% given 300 mg/m2 daily, while adverse side effects were common and ALT or AST elevations of 2.5 to 5 times ULN occurred in 10%).
- Esteva FJ, Glaspy J, Baidas S, Laufman L, Hutchins L, Dickler M, Tripathy D, et al. Multicenter phase II study of oral bexarotene for patients with metastatic breast cancer. J Clin Oncol 2003; 21: 999-1006. [PubMed: 12637463](Among 145 women with metastatic breast cancer treated with bexarotene, clinical responses were uncommon and usually partial, while adverse events included hypertriglyceridemia [84%], dry skin [34%], weakness [30%] and headache [27%] and two patients [1%] had dose limiting liver test elevations).
- Smit JV, Franssen ME, de Jong EM, Lambert J, Roseeuw DI, De Weert J, Yocum RC, et al. A phase II multicenter clinical trial of systemic bexarotene in psoriasis. J Am Acad Dermatol 2004; 51: 249-56. [PubMed: 15280844](Among 50 patients with severe psoriasis treated with bexarotene in 4 doses for 12-24 weeks, clinical responses occurred in 22-52% of patients and adverse events included hypertriglyceridemia [56%], decrease in serum free T4 levels [54%], fatigue [14%], pruritus [14%] and ALT elevations [6%]).
- Kannangara AP, Levitan D, Fleischer AB Jr. Evaluation of the efficacy of the combination of oral bexarotene and methotrexate for the treatment of early stage treatment-refractory cutaneous T-cell lymphoma. J Dermatolog Treat 2009; 20: 169-76. [PubMed: 19016373](Among 12 patients with refractory CTCL treated with methotrexate and bexarotene, partial responses occurred in 58%, but one patient who had received 13 g of methotrexate developed grade 3 “liver failure”).
- Väkevä L, Ranki A, Hahtola S. Ten-year experience of bexarotene therapy for cutaneous T-cell lymphoma in Finland. Acta Derm Venereol 2012; 92: 258-63. [PubMed: 22678563](Description of 37 Finnish patients with CTCL treated with bexarotene focusing on management of adverse reactions including hypertriglyceridemia and hypothyroidism; mentions that 4 patients [11%] had ALT or AST elevations above 5 times ULN, but the levels returned to normal upon stopping, although they recurred in 1 of 2 patients who restarted therapy at a lower dose).
- Whittaker S, Ortiz P, Dummer R, Ranki A, Hasan B, Meulemans B, Gellrich S, et al. Efficacy and safety of bexarotene combined with psoralen-ultraviolet A (PUVA) compared with PUVA treatment alone in stage IB-IIA mycosis fungoides: final results from the EORTC Cutaneous Lymphoma Task Force phase III randomized clinical trial (NCT00056056). Br J Dermatol 2012; 167: 678-87. [PubMed: 22924950](Among 87 patients with mycosis fungoides treated with psoralen with ultraviolet A [PUVA] with or without bexarotene, overall response rates were similar [77% vs 71%], but side effects were greater with the combination including ALT elevations above 5 times ULN [8.7% vs 0%]).
- Lerner V, Miodownik C, Gibel A, Sirota P, Bush I, Elliot H, Benatov R, et al. The retinoid X receptor agonist bexarotene relieves positive symptoms of schizophrenia: a 6-week, randomized, double-blind, placebo-controlled multicenter trial. J Clin Psychiatry 2013; 74: 1224-32. [PubMed: 24434091](Among 90 patients with schizophrenia treated with bexarotene [75 mg daily] or placebo for 6 weeks, serum ALT and AST levels did not change significantly in either group).
- Hamada T, Sugaya M, Tokura Y, Ohtsuka M, Tsuboi R, Nagatani T, Tani M, et al. Phase I/II study of the oral retinoid X receptor agonist bexarotene in Japanese patients with cutaneous T-cell lymphomas. J Dermatol 2017; 44: 135-42. [PubMed: 27543197](Among 16 patients with cutaneous T cell lymphomas treated with bexarotene for 24 weeks, ALT elevations were reported in 4 [25%] and led to early discontinuation in 1 [6%].
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Bexarotene Reduces Production of CCL22 From Tumor-Associated Macrophages in Cutaneous T-Cell Lymphoma.[Front Oncol. 2019]Bexarotene Reduces Production of CCL22 From Tumor-Associated Macrophages in Cutaneous T-Cell Lymphoma.Tanita K, Fujimura T, Sato Y, Lyu C, Kambayashi Y, Ogata D, Fukushima S, Miyashita A, Nakajima H, Nakamura M, et al. Front Oncol. 2019; 9:907. Epub 2019 Sep 20.
- Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action.[Clin Cancer Res. 2002]Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action.Zhang C, Hazarika P, Ni X, Weidner DA, Duvic M. Clin Cancer Res. 2002 May; 8(5):1234-40.
- Comparison of the Efficacy and Safety of Bexarotene and Photo(Chemo)Therapy Combination Therapy and Bexarotene Monotherapy for Cutaneous T-Cell Lymphoma.[Dermatol Ther (Heidelb). 2022]Comparison of the Efficacy and Safety of Bexarotene and Photo(Chemo)Therapy Combination Therapy and Bexarotene Monotherapy for Cutaneous T-Cell Lymphoma.Morita A, Tateishi C, Ikumi K, Hayashi D, Nakada A, Nishihara H, Torii K, Nishida E, Tsuruta D. Dermatol Ther (Heidelb). 2022 Mar; 12(3):615-629. Epub 2022 Jan 27.
- Review Bexarotene.[Am J Clin Dermatol. 2000]Review Bexarotene.Lowe MN, Plosker GL. Am J Clin Dermatol. 2000 Jul-Aug; 1(4):245-50; discussion 251-2.
- U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma.[Br J Dermatol. 2013]U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma.Scarisbrick JJ, Morris S, Azurdia R, Illidge T, Parry E, Graham-Brown R, Cowan R, Gallop-Evans E, Wachsmuth R, Eagle M, et al. Br J Dermatol. 2013 Jan; 168(1):192-200. Epub 2012 Dec 3.
- Bexarotene - LiverToxBexarotene - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...