NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Dean L, McEntyre J, editors. Coffee Break: Tutorials for NCBI Tools [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 1999-.
An effective malaria vaccine may well be on the way. These days, vaccines are a normal part of our lives. Most United States children are vaccinated for some form of hepatitis, along with a slew of other diseases including polio, rubella, measles, and mumps. Some of us take these things for granted, but in other parts of the world, vaccines are not so easily obtained.
Malaria, globally one of the most devastating diseases affecting humans, is caused by four species of the Plasmodium genus. Of the four species that can cause malaria in humans, Plasmodium falciparum is the one that causes most deaths (1). Malaria could be made significantly less deadly by using a cheap, easy-to-produce vaccine.
Traditionally, vaccines have been made from attenuated viruses or bacteria, or by creating a virus-like particle, in the case of some types of flu vaccine(2). Another method for creating vaccines, recently becoming more popular, is to create a recombinant protein. Vaccines made with recombinant proteins offer an advantage over other types of vaccines in that there is no need to handle the actual disease-causing agent, which can be costly and sometimes dangerous. Instead, one or more proteins are expressed and purified, then formulated to be injected into the subject to cause an antibody response against the foreign protein. If, in the future, that person is again exposed to the same protein, it is hoped that his or her immune system will recognize it more quickly as a threat. Recombinant protein vaccines are currently being researched and tested for a variety of diseases, including ricin toxin exposure, pneumococcus infection, and malaria (3-5). There are currently a few different recombinant protein vaccines against P. falciparum being tested in clinical trials, including apical membrane antigen 1 (AMA1) (6).
AMA1 appears on the surface of the merozoite during the blood-stage of P. falciparum parasites (Figure 1). Studies suggest that AMA1 is a necessary component for invasion of red blood cells by merozoites (7). Vaccination with recombinant AMA1 has been shown to elicit antibody responses that provide protection against homologous parasite challenges in both rodent and monkey models of malaria infection, and a derivative vaccine has been in a Phase I human trial in Mali, West Africa (7-9).
How did we arrive at this point? As early as 1997, scientists were testing some form of AMA1 for its antibody response against Plasmodium species (7). But to use a protein as a vaccine, it must be economically feasible to create large amounts of the protein. The Plasmodium genome is highly A+T rich, which makes it hard to express P. falciparum proteins in sufficient yields for commercial use in classic expression systems such as Escherichia coli and Pichia pastoris. One way to augment expression in such species is to recode the gene to match the host’s tRNA pool. DNA codons that are rare in the target species are replaced with those that are used more often, while keeping the amino acid sequence unchanged. This raises protein yield because more tRNA molecules exist in the cell for those codons, making protein synthesis easier.
Genes that are recoded, or "synthetic" have been used for years to raise yields and reduce costs for many medically and industrially useful proteins, such as insulin (10). For AMA1, Pichia pastoris is the most widely used expression system, because the protein can be expressed in much greater quantities than in the original host organism (8, 9).
One of the problems with AMA1, however, is that it is strain specific. This means that an AMA1 protein cloned from one strain of P. falciparum, the FVO strain, for example, may not protect against other strains of P. falciparum, such as the 3D7 strain (8). This is because of a highly polymorphic cluster of amino acids surrounding the interior of the protein (Figure 2 ).

Figure 2
(Top) Three-dimensional view of the AMA1 protein with domains colored differently. You can download the 3-dimensional model in  . (Bottom) Three-dimensional view of the AMA1 protein with polymorphic residues highlighted.
. (Bottom) Three-dimensional view of the AMA1 protein with polymorphic residues highlighted.
To remedy this, some AMA1-derived vaccines, such as AMA1-C1, are mixtures of AMA1 cloned from different strains of P. falciparum (9). These combination vaccines are intended to elicit better antibody responses against diverse strains of P. falciparum than any one strain-specific AMA1 protein (11).
Manufacturing a cheap, effective vaccine for malaria will depend on many factors. A large-scale method for preparing AMA1-derived vaccines is still far from a reality, and limited human trial data is available (9). There are still three other species of Plasmodium that can cause malaria, so effective vaccines must be considered for these, especially because it has been shown that P. vivax can replace P. falciparum in areas in which the falciparum species has been contained (6). Interest has also been shown in certain oligodeoxynucleotide (ODN) molecules that, when added to the vaccine formulation, may strengthen the immune response against AMA1-derived vaccines (11).
Protein vaccines, as compared to other types of vaccines, could potentially be cheap, easy to produce vaccine candidates against malaria, one of the world's deadliest diseases. Promising early research results have been shown, but much research must still be done to make a malaria vaccine a reality. Clinicians and researchers have their work cut out for them, as always. For now, effective treatment is still extremely important in the fight against devastating diseases such as malaria, but in the next few years, that may change.
This Coffee Break was contributed by Tyler Beck, during an internship at the National Center for Biotechnology Information, while on sabbatical from the University of Maryland, Baltimore County.
References
- 1.
- Jamison DT , Breman JG , Measham AR , Alleyne G , Claeson M , Evans DB , Jha P , Mills A , Musgrove P . , editors.21. Conquering Malaria. In: Disease control priorities in developing countries. Washington (DC):IBRD/The World Book and Oxford University Press; 2006 (Bookshelf) [PubMed: 21250338]
- 2.
- Galarza JM , Latham T , Cupo A . Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18(1):244–251. [PubMed: 15802970]
- 3.
- Jomaa M , Terry S , Hale C , Jones C , Dougan G , Brown J . Immunization with the iron uptake ABC transporter PiaA and PiuA prevents respiratory infecation with Streptococcus pneumoniae. Vaccine. 2006;24(24):5133–5139. [PubMed: 16707196]
- 4.
- Vitetta ES , Smallshaw JE , Coleman E , Jafri H , Foster C , Munford R , Schindler J . A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A. 2006;103(7):2268–2273. [PMC free article: PMC1413738] [PubMed: 16461456]
- 5.
- James S , Miller L . Malaria vaccine development: status report[monograph online]. Bethesda (MD): National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH); 2001. [updated 2001 Jan. 5; cited 2006 Jun 21]. Available from: http://www
.niaid.nih .gov/dmid/malaria/malariavac.html. - 6.
- Matuschewski K . Vaccine development against malaria. Curr Opin Immunol. 2006;18(4):449–457. [PubMed: 16765576]
- 7.
- Amante FH , Crewther PE , Anders RF , Good MF . A cryptic T cell epitope on the apical membrane antigen 1 of Plasmodium chabaudi adami can prime for an anamnestic antibody response. J Immunol. 1997;159(11):5535–5544. [PubMed: 9548494]
- 8.
- Kennedy MC , Wang J , Zhang Y , Miles AP , Chitsaz F , Saul A , Long CA , Miller LH , Stowers AW . In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70(12):6948–6960. [PMC free article: PMC133034] [PubMed: 12438374]
- 9.
- Malkin EM , Diemert DJ , McArthur JH , Perreault JR , Miles AP , Giersing BK , Mullen GE , Orcutt A , Muratova O , Awkal M , Zhou H , Wang J , Stowers A , Long CA , Mahanty S , Miller LH , Saul A , Durbin AP . Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73(6):3677–3685. [PMC free article: PMC1111886] [PubMed: 15908397]
- 10.
- Dunn CJ , Plosker GI , Keating GM , McKeage K , Scott LJ . Insulin glargine: an updated review of its use in the management of diabetes mellitus. Drugs. 2003;63(16):1743–1778. [PubMed: 12904090]
- 11.
- Mullen GED , Giersing BK , Ajose-Popoola O , Davis HL , Kothe C , Zhou H , Aebig J , Dobrescu G , Saul A , Long CA . Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24(14):2497–2505. [PubMed: 16434128]
- Natural gene/synthetic gene comparison using BLAST2
- Natural protein/synthetic protein comparison using BLAST2
- Taxonomy record for Plasmodium falciparum
- Gene record for P. falciparum ama1
- Clinical trial data from Clinicaltrials.gov
- The Malaria Vaccine Initiative homepage
- NIH News: NIAID and the Malaria Vaccine Initiative/PATH Sign Agreement to Accelerate Malaria Vaccine Research
- See a map of malaria endemicity from World Malaria Report 2005
- AAAS: Malaria and Development in Africa: A Cross-Sectoral Approach
- The Synthetic Gene Database homepage
- Will malaria soon be a thing of the past? - Coffee BreakWill malaria soon be a thing of the past? - Coffee Break
Your browsing activity is empty.
Activity recording is turned off.
See more...

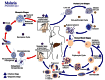
 . Sporozoites infect liver cells
. Sporozoites infect liver cells  and mature into schizonts
and mature into schizonts  , which rupture and release merozoites
, which rupture and release merozoites