This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
Deficits in end-of-life (EOL) care in nursing homes (NHs) have been reported, but the impact of NH-based palliative care teams (PCTeams) has been unstudied and untested.
Objective:
To assess the effectiveness of NH PCTeams on resident outcomes and care processes.
Methods:
The study team enrolled 31 NHs in upper New York State (NYS) in a cluster randomized controlled trial (RCT). The intervention components included team development, staff training in EOL care, and PCTeam activation and rounding with a nurse interventionist. Using the Minimum Data Set and Vital Status files, we developed 4 risk-adjusted outcome measures: place of death, number of hospitalizations, self-reported moderate-to-severe pain, and depressive symptoms, all within 90 days of death. Staff surveys measured care processes. Surveys with family members of decedent residents assessed satisfaction with care. To understand any challenges that staff may have experienced during the intervention, we conducted in-depth interviews in all treatment-arm NHs.
For each outcome a difference-in-difference model compared the preintervention and postintervention periods using logistic and Poisson regressions with random effects to account for patient clustering. The analyses included 2 control groups: facilities recruited for the RCT and randomized to the control arm, and all other NYS facilities that did not participate in the study. We also conducted a sensitivity analysis comparing treatment arm NHs in which PCTeams were or were not consistently employed (referred to as working or nonworking teams, respectively). We classified teams as working or not based on analysis of in-depth interviews with staff in the treatment arm NHs. We compared facilities with working and nonworking teams with each other and with the randomized and nonrandomized controls on all outcomes. We employed a generalized linear model with facility random effects.
Results:
In total, 14 treatment and 11 control NHs completed the RCT. The analytical sample included 5830 decedents from the RCT-participating homes and 119 486 from all other facilities (n = 609) in NYS. We obtained preintervention surveys from 1018 staff in all participating NHs. We completed in-depth interviews with 41 staff in treatment homes after the intervention. These interviews revealed that only 6 of the 14 facilities had consistently working PCTeams throughout the study period. These working teams were characterized by a clear and shared mission, a sense that the team influenced residents' care, and a perception of continued team sustainability. In the main analysis we found no statistically significant effect of the intervention; however, based on the sensitivity analyses, decedents in homes with working teams had significant reductions in the odds of in-hospital death compared with homes with nonworking teams (odds ratio [OR], 0.400; P < .001), control (OR, 0.482; P < .05), and nonrandomized control NHs (0.581; P < .01). Decedents in these NHs had reduced rates of depressive symptoms (OR, 0.191; P < .05), but not pain or hospitalizations. We did not evaluate care processes and satisfaction with care among family members due to insufficient data.
Conclusions:
Overall, we found no statistically significant impact of the intervention on patient outcomes; however, sensitivity analysis suggests that some treatment-arm homes may have been at least partially successful in influencing EOL care quality.
Limitations:
Some NHs may have been better equipped to implement PCTeams than others from the beginning. We were not able to identify this difference at baseline.
Background
The 2014 Institute of Medicine (now known as the National Academy of Medicine) report on end-of-life care identified communication skills, interprofessional collaboration, and symptom management to be key palliative care (PC) competencies required of providers caring for individuals with advanced illness.1 Nursing homes (NHs), which care for frail and severely ill residents, and where more than 30% of Americans die,2,3 largely underperform on these competencies.4,5 Studies attest to insufficient management of symptoms including pain,6,7 frequent and often unnecessary hospitalizations,8,9 shortcomings in teamwork and communication,10-12 and inadequate PC knowledge and skills among NH staff.13,14
At the same time, research focusing on health care teams has demonstrated that skills such as communication and interprofessional collaboration are the hallmark of effective teams, driving both quality and improved patient outcomes. Studies of NHs have shown that better teamwork among staff and improved communication between staff and residents/family members were associated with higher overall quality of care12,15 and better patient outcomes,16 including those at the end of life (EOL).11,17
To date, several models for EOL care delivery in NHs have been employed, including the use of hospice, which cares for EOL patients in specialized PC units, and the use of PC consulting services.18 Hospice enrollment has been shown to reduce the likelihood of terminal hospitalizations19 and is associated with better pain management—but not always with better management of other symptoms.20 The research team's own research in this regard, based on >2 million NH decedents in more than 16 000 NHs (years 2003-2007) has shown that the use of hospice in the last 30 days of life lowers the odds of in-hospital death by 95% (CI, 0.050-0.052).21 However, integration of hospice into NHs has been very difficult, due to conflicting financial incentives and barriers to referral, which are often exacerbated by poor recognition of terminal illness by the NH staff.22,23 Furthermore, hospice benefit payments require an assumption of life expectancy shorter than 6 months and an agreement to forgo curative treatment for the terminal condition; thus, perhaps, it is not surprising that hospice still plays a limited role in the care of patients living in NHs. Although 80% of NHs report having contracts with hospice providers,24 fewer than a third of NH decedents receive hospice care in the last 30 days of life, and most of this care is received just days before death.25
There are only a few examples of PC units in US NHs, and evidence of their effectiveness has been very limited.26 The presence of PC teams (PCTeams) in NHs has been reported as having a positive impact on hospice enrollment, advance care planning discussions, and pain assessment; however, these findings are based on a single study of 7 facilities, and several serious study limitations make the results unreliable.27 A meta-analysis of 19 studies from the United Kingdom demonstrated that patients with serious, life-limiting illness who receive care from home and hospital-based PCTeams have significantly better outcomes for pain and other symptom management.28 Similar evidence for the impact of hospital-based PCTeams in the United States also exists.29-38 However, to our knowledge, there have been no comparative randomized controlled trials (RCTs) of PCTeams in NHs—in the United States or elsewhere. PC provision, either through contractual arrangements with external teams or through facility-based PCTeams, is only sporadically available.39,40 Thus, to date, the impact of PCTeams on EOL outcomes among NH residents has been largely unstudied and untested.
Several educational interventions designed to improve care at the EOL in NHs have been previously implemented and evaluated.41 Interventions that focused on educational programs to improve staff knowledge in EOL care have reported positive results in staff competency but have not examined the effect of the intervention on residents' outcomes.42 Other quasi-experimental, educational interventions demonstrated significantly greater control of pain and dyspnea in the intervention homes compared with the controls.43,44 A large RCT of quality improvement interventions in 113 NHs, not focusing specifically on palliative or EOL care, demonstrated that simply providing staff education is not enough to bring about measurable improvements in quality of care.45 Overall, evaluations of the impact of educational materials have suggested that when such materials are used alone, they do not appear to impact patient outcomes compared with no intervention, but when introduced as part of practice change they are effective.46,47
To address these gaps in the literature and in NH practice, we implemented an intervention (rooted in the conceptual model presented in Section 3.2, below) titled Improving Palliative Care Through Teamwork (IMPACTT), which, to our knowledge, was the first NH-level RCT sufficiently powered and designed to evaluate the impact of NH-based PCTeams. Specifically, we proposed to test the following hypotheses:
- H1. Decedent residents in NHs with PCTeams, compared with residents who receive standard care, have better EOL risk-adjusted outcomes and care processes regarding:
- Pain
- Depression
- In-hospital deaths
- Hospitalizations
- Advance directives
- H2. Direct care staff in NHs with PCTeams, compared with those involved in standard care, achieve better EOL processes and outcomes, measured by the following:
- Perceived PC competency (EOL-specific domains of assessment, delivery, communication with residents/families)
- Communication/coordination among providers
- Teamwork effectiveness
- Staff satisfaction
- H3. Family caregivers of deceased residents in the intervention NHs, compared with those in the control, report receiving more patient- and family-centered care, as measured by higher levels of satisfaction with the following:
- Shared decision making between providers, the patient, and the family
- Care that is respectful of the patient wishes and dignity
- Attention to the emotional and spiritual needs of the family
Participation of Patients and other Stakeholders
Existing literature on palliative/EOL care has commented on the discrepancies in perceptions between NH leaders, who believe they are delivering high-quality care, and the experiences reported by residents and family members. Through the involvement of multiple stakeholders, with whom we exchanged ideas and expectations about the goals of care and the best ways to reach these goals, we aimed to create shared understanding of the key dimensions of high-quality EOL care. We achieved this by involving NH residents, their family members, clinicians who care for them (physicians, nurses, licensed practical nurses [LPNs], certified nursing assistants [CNAs], and social workers), local/regional health system planners, representatives of state NH associations, and policy experts.
In Table 1, we identify all stakeholders, the methods used to recruit them, and the numbers of stakeholders participating in each group throughout the study period. Methods for stakeholder involvement are described below.
Table 1
Stakeholder Engagement by Type: Identification, Engagement Type, and Number.
NH Residents and Family Caregivers
We invited residents and family caregivers to participate in semistructured interviews (conducted before active intervention phase), through which we elicited their expectations for high-quality PC, and what was important to them, and identified additional areas for improvements in care delivery processes and outcomes. Residents and their family members were approached by NH staff, usually the social worker, and asked if they would like to speak to a member of our research team. The semistructured interview questions used in this phase of stakeholder engagement are presented in Appendices 9.2 and 9.3. Residents and family members were largely unfamiliar with the term palliative care but clearly identified aspects of care delivery that were palliative in nature and were aligned with their values and expectations for care, including an emphasis on care that honored their individuality and personal achievements, care preferences, and preferred communication frequency and style. We used findings from these interviews to inform the intervention.48
NH Clinical Staff
Before the intervention began, we employed 2 rounds of a Delphi process involving clinical and administrative staff from the treatment homes. We did this to arrive at a consensus in determining PC and EOL best practice standards to be used across all intervention facilities and to serve as the template for how to implement PC in the NH setting. Specifically requesting the input of clinicians, we invited 48 NH leaders to review the importance (10-point Likert scale) and the feasibility (5-point Likert scale) of these potential standards and practices. Overall, these 48 NH leaders converged on 17 PC best practice standards that were identified for use by NH PCTeams as being both important and feasible in the long-term care setting. These best practices were distributed over 7 domains of care: structure and process, physical, psychosocial and psychiatric, social, cultural, EOL, and ethical and legal. In addition, several standards for NH PCTeam structure and operations were also defined, including team referrals and screening, frequency of rounding, communication with residents and other staff members, staff education, and bereavement and grief support. Five disciplines (social work, certified nurse assistant, nurse, physician, and nurse practitioner or physician assistant) were identified as comprising an ideal core team, and 3 other disciplines were proposed as extended or ad hoc members (therapists, chaplains, and dietary staff). We then used these 17 best practices as a template for practical direction to guide PCTeam processes and operations specific to this care setting (see Appendix 9.6 for a complete list of these Preferred Palliative Care Practices for Nursing Homes.)40 This process helped ensure transparency of the project, increased the likelihood that the resulting operational recommendations were relevant, and improved the project's chances of being adopted into practice.
Stakeholder Advisory and Validation Group
The Stakeholder Advisory and Validation Group (SA&VG) consisted of invited local patient advocates: representatives of hospice agencies; an advocacy group for disability rights; staff from a local Health System Agency; members of a statewide NH association; and recognized state and national experts in PC, EOL care, and Strategies & Tools to Enhance Performance and Patient Safety (TeamSTEPPS). The SA&VG met 4 times during the project: (1) twice in person with members of our research team and stakeholders from our treatment facilities and (2) twice through webinars. We solicited their thoughts and feedback for implementing the intervention as well as supporting the developing PCTeams. Their feedback affirmed the importance of this study and its objectives; to an extent, it also predicted the overall finding that some facilities would be more successful at launching and sustaining a PCTeam than others. Specifically, our SA&VG suggested that the key critical resources would include time, especially time for staff to learn to work as a team; leadership support and a clear vision that PC is a priority; and staffing issues, including managing turnover.
Methods
Study Overview
We launched an intervention study to examine the effectiveness of NH PC best practices, implemented through integrated NH PCTeams, in improving the quality of care processes and outcomes for residents at the EOL. We used a facility-level (ie, cluster) RCT design and a difference-in-difference (DID) analytic approach to test our outcome measures. Following facility-level PCTeam building and palliative/EOL staff educational sessions (preintervention), we implemented in each treatment facility a 2-month active intervention phase (team rounding with a nurse interventionist), immediately followed by an 8- to 10-month passive intervention phase, when PC experts were available for consultation as needed, on demand. We employed the DID analytical approach to determine the impact of the intervention on patient outcomes (resident death in hospital, hospitalizations in the past 6 months of life, self-reported pain, and self-reported depression). We also conducted staff surveys to assess the impact of the intervention on care processes, and postbereavement surveys with family members to assess the impact on patient-centered care. Furthermore, to understand how elements of the intervention were conducted in each facility, we conducted semistructured interviews with treatment NH staff at the end of the passive intervention phase.
The study was registered at ClinicalTrials.gov (identifier NCT01990742), and it was reviewed and approved by the IRB at the University of Rochester.
Study Design, Cohort, and Setting
We designed a facility-level multicomponent intervention strategy that included building NH-based PCTeams, providing existing NH staff with palliative and EOL geriatric training, and team activation and rounding with a nurse interventionist.
The conceptual framework on which the intervention is based (Figure 1) integrates 3 domains: (1) organizational capacity (eg, readiness for change, facility resources); (2) care processes (eg, teamwork), and (3) resident and staff outcomes (eg, adverse symptoms, staff satisfaction).
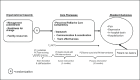
Figure 1
Conceptual Framework and Study Design.
Organizations and their leaders play an important role in the uptake of new knowledge, its adoption, and its application into practice.49,50 Their support and participation are necessary to create practice change and to secure adequate facility-level resources to sustain it. We employed rapid ethnographic assessments (REAs) to understand barriers to the intervention implementation and how the NH leadership dealt with the challenges encountered.
Prior research has identified the importance of care processes such as assessment and management of PC symptoms, communication and coordination of care among providers, and communication between providers and residents/families.4 Residents in NHs with better team and PC processes have been shown to have better outcomes, including those at the EOL.11,17
To assess the impact of the intervention on residents' outcomes, we identified 4 risk-adjusted quality measures: place of death (in an NH or a hospital), number of hospitalizations at EOL, self-reported pain, and self-reported depression. These are considered to be meaningful indicators of quality because they meet the following criteria: (1) address an outcome of importance to residents; (2) can be affected by clinical care provided; (3) are calculated on a sufficiently large number of residents within a facility to be statistically meaningful; and (4) when adjusted for residents' risks account for factors over which the NH has no control.51-53 Before the onset of the study we assessed face validity of the proposed outcome measures in a focus group of caregivers whose family members were in an NH. During the study period the proposed outcome measures were further discussed with stakeholders who represented NH staff and patient advocates.
Detailed information on the study design, recruitment and retention, intervention design, data sources, and baseline outcomes has been presented in a prior published paper.54 Below, we provide a brief summary of the relevant information.
In the RCT, known as IMPACTT, we used a mixed methods research design with a DID analytic technique. All freestanding NHs in the metropolitan areas of Syracuse, Rochester, and Buffalo were approached for recruitment (n = 136). This included facilities in both urban and rural areas. A letter of invitation was issued to these NHs before we applied to PCORI for funding. The letter included a brief description of the study, outlining data collection requirements, estimated time commitment needed from various staff members, and the potential benefits of participation to NHs. A letter of commitment from the facility administrator was required to demonstrate commitment to participate. After learning that the study was to be funded by PCORI, we “re-recruited” all the facilities, since at least a year had elapsed since the initial recruitment.
Based on power calculations, a sample size of 30 facilities was sufficient for the RCT.54 We enrolled the first to respond to our renewed invitation; we stopped enrolling once 32 facilities agreed to participate.
Participants
We based our primary outcomes on resident-level assessment data derived from the Minimum Data Set (MDS) 3.0 (discussed in more detail below, in Section 3.6) obtained for all NHs in New York State (NYS). Using the MDS, we developed risk-adjusted outcome measures for NH decedents in the intervention homes (both treatment and control) and in all other NHs in NYS (not participating in the RCT). This latter “nonrandomized” control group was included to test for any temporal effects.
The analytical sample included decedent Medicare beneficiaries aged 65+ who had been residents in NYS NHs, died there or were discharged to an acute care hospital where they died within 18 days (90% of residents who died in the hospital die within this time frame), between October 1, 2012, and April 30, 2016. Specifically, this group included 5830 decedent residents from 25 study facilities (14 intervention and 11 controls) and 119 486 decedents from the remaining 609 facilities in the state—that is, the nonrandomized control group.
Interventions and Follow-up
A facility-level intervention, IMPACTT involved a multicomponent strategy that included implementing facility-based PCTeams. To aid in the development of these teams, we deployed TeamSTEPPS (see https://www.ahrq.gov/teamstepps/index.html). This development strategy was created by the Department of Defense and the Agency for Healthcare Research and Quality. This model has been tested in more than 2 decades of research and has demonstrated effectiveness in acute health care settings.55,56
We provided staff in all treatment NHs with a template for PCTeam operational guidelines, which were developed from the consensus building of the Delphi process described above.40 These guidelines suggested that PCTeams should be interdisciplinary and include nurses, certified nursing assistants, social workers, chaplains, nurse practitioners, and physicians; they further suggested that teams meet regularly, recommending at least a weekly conference. Other ideas included how to communicate with other stakeholders (eg, hospice agencies, physicians), interactions with family members, and processes for following through with the plan of care. These suggestions were presented as recommended guidelines, not as requirements, as it was clear that each NH had its own processes and would not respond positively to requirements arbitrarily imposed by outsiders.
Staff members in the treatment NHs were also provided with palliative and EOL geriatric training (End-of-Life Nursing Education [ENLEC] curriculum; see https://www.reliaslearning.com/elnec). ELNEC-geriatric is based on the Palliative Care Educational Resource Team program, which has been shown to be effective in increasing knowledge, skills, and confidence related to EOL care.57 Team development and staff training in the treatment NHs were followed by a 2-month-long active intervention phase during which a gero-PC nurse practitioner interventionist (from the research team) rounded with the teams as they saw and/or discussed residents' care. The frequency of rounds with the interventionist was at the discretion of each NH, but the interventionist made, at a minimum, weekly contacts with each team during the active intervention phase.
A passive phase of at least 8 months immediately followed, during which the nurse interventionist was available to further coach the team on an as-needed/requested basis. Following the active intervention phase, each treatment NH received free online access to the ELNEC modules for a period of 3 years, allowing it to continue with the training for any new staff members. The passive phase was initially scheduled to last 10 months but was shortened to 8 months for those facilities that experienced significant implementation delays. Of the 14 treatment homes, only 5 underwent the shorter passive phase. The control homes did not receive any intervention activities.
Several aspects of the data collection (described below in detail) exceeded the period of exposure to the intervention. This was part of the design of the intervention as originally proposed and included the collection of postintervention surveys among NH staff, family bereavement surveys, and the conduct of the REAs.
Study Outcomes
The study's primary outcomes measured EOL quality of care. The outcomes of interest were place of death (NH vs hospital), number of hospitalizations, self-reported moderate-to-severe pain, and depressive symptoms, all within 90 days of death. We focused on these outcomes because they can be affected by NH staff ability to assess symptoms, coordinate care, communicate with the residents and their family members, and deliver care to the residents— in other words, the key PC competencies being tested in this intervention. Furthermore, patients and their families consider these outcomes important.
In employing these outcomes, we followed the methodology we developed and reported on previously.53 Obtained from the MDS data, we defined primary outcomes as place of death = 1 if death occurred in a hospital, 0 otherwise; number of hospitalizations within the last 90 days of stay (excluding last hospital stay if death occurred in a hospital); self-reported pain = 1 if reported as moderate to severe, 0 otherwise; and depression = 1 if reported/displayed by the residents, 0 otherwise. We used discharge and readmission records to calculate death in hospital and hospitalization. We used the last assessment before death to calculate the pain and depression outcomes. Resident risk factors (see Supplemental Tables S.1 and S.2) were obtained from the last assessment before death or were imputed from a prior assessment when necessary. Because all measures of interest represent outcomes that residents would prefer to avoid (ie, negative), lower values should be interpreted as better quality.
The secondary outcomes of interest, obtained from staff surveys, were 5 care process measures based on staff perceptions of their team's cohesion, communication/coordination, perceived team performance, and PC competency, and their organization's readiness to implement PC. We measured each care process as a score on a Likert scale ranging from 1 (worse) to 5 (best). Our prior studies showed that the tools used to assess care process domains were psychometrically reliable and conceptually valid.58,59 We have also previously demonstrated a relationship between care process measures and residents' EOL outcomes.11
Data Sources and Analytical Samples
We employed multiple sources of data to evaluate the impact of the intervention.
Secondary Data
To assess the impact of the intervention on residents' outcomes (hypothesis 1), we used the MDS 3.0 and vital status data. The MDS 3.0 is used by the Centers for Medicare & Medicaid Services (CMS) to create Nursing Home Compare Quality Measures and 5-star ratings, as well as for Medicare and Medicaid payment systems.
We obtained the MDS 3.0 and the Vital Status File (VSF) data sets under a data use agreement with the CMS. We merged MDS 3.0 data with the VSF and longitudinally for all NH residents in NYS for the period of October 1, 2012, through September 30, 2013 (preintervention), and for October 1, 2013, through April 30, 2016 (intervention period). The VSF contains date of birth, date of death, and demographic information for each beneficiary ever entitled to Medicare. We linked the files using a BENE_ID (VSF identifier) to RES_ID (MDS identifier) crosswalk as well as with facility-level identifications.
The MDS 3.0 is a resident-level database that contains screening, clinical, and functional status elements completed for all residents at admission, quarterly, and annually, and when there is a significant change in status. The MDS includes common definitions and coding categories, which are the foundation of a comprehensive assessment for all residents of NHs certified to participate in Medicare or Medicaid (https://downloads.cms.gov/files/MDS-30-RAI-Manual-v115-October-2017.pdf). Trained NH personnel completed all assessments. Some missing items on resident-level health assessments in the MDS for each outcome are expected for several reasons: An item may not be relevant for a resident due to his or her clinical status. The assessor may not have necessary information at assessment time, and not all health assessments contain all items collected in the MDS. To reduce missing data, we limited risk factors to items that are available on the prospective payment system and quarterly assessments. If a resident had missing items on his or her final health assessments which were considered risk factors in our models, we attempted to impute the information from a prior comprehensive assessment. If imputation was not possible (eg, the condition was acute), we excluded the resident from the risk-adjustment model. To account for statistical uncertainty due to missing data, we conducted several sensitivity analyses at those stages when problems due to missing data may have arisen.53
Primary Data
To measure the impact of the intervention on care processes (hypothesis 2), we conducted primary data collection via staff surveys with all staff providing direct patient care (eg, physicians, nurses, nurse assistants, therapists, social workers). We conducted the surveys before the start of the intervention and again once it was completed.
We based all measures of care process domains employed in the survey on psychometrically tested tools that were extensively examined in prior studies,4,59 and that were revalidated on the data from the surveys implemented in the treatment and control homes of the IMPACTT intervention.54 The survey tool and the survey implementation process are described in detail in a prior study.54 Project staff provided all NH leaders/liaisons with survey packets and a request to distribute these materials to all staff with direct care responsibilities. Completed surveys were mailed directly to project staff in prepaid and preaddressed envelopes provided with the survey. The key care process measures of interest included in this survey were team communication/coordination, perceived team effectiveness, PC competency, team cohesion, and organizational readiness for PC. Each domain is measures on a Likert scale (1-5), with higher values indicating more positive appraisals. A copy of this survey is included in Appendix 9.2.
We obtained the preintervention staff surveys from 1018 staff respondents (response rate of 30%) in all participating facilities. The response rate to postintervention surveys was considerably lower (n = 466; response rate = 21%), with 3 treatment and 9 control homes not participating at all. This precluded us from conducting statistical analyses comparing treatment and control facilities regarding the intervention's impact on palliative and EOL care processes (hypothesis 2). However, we conducted statistical analyses on care process measures in the sensitivity analysis (described below) on a smaller subset of survey responses available from the treatment NHs (ranging from N = 911-965, depending on the measure).
To examine the domains of patient- and family-centered care (hypothesis 3), we used an abbreviated version of the After-death Bereaved Family Member Survey, a component of the Toolkit of Instruments to Measure End-of-Life Care (TIME), which has been developed and extensively tested for validity and reliability, including in NHs.60 This survey measures domains of shared decision making and the emotional and spiritual needs of the family. Each domain is measured as a Likert scale score, with a higher score indicating more opportunity to improve. All facilities were asked to mail an abbreviated TIME survey to the family member identified as the contact person no earlier than 1 month but no later than 3 months following a resident's death. The family members were asked to return the survey, anonymously, directly to the research team in a prepaid envelope provided. Because the research team did not have information about the deceased residents' family members' names and addresses, it could not contact families directly to solicit their responses; instead it relied on the NHs to address the survey packets and distribute them via mail. The responses to 2 waves of the TIME survey were very disappointing: We received only 238 responses in the preintervention phase, and only 14 families returned surveys after we had provided 625 survey packets to facilities for distribution to deceased residents' families during the postintervention phase (2.2% response rate). Because of this low response rate, we could not conduct the analyses related to hypothesis 3.
We also conducted REAs in all treatment facilities. The REAs are a set of techniques that include interviewing and field observations used for rapid acquisition of data that are rich in work experiences of the subject population.61 Data for our REAs contained semistructured interviews with staff (N = 41), field notes, and the collection of written materials. We conducted the REAs postintervention in order to identify barriers/challenges to PCTeam development and sustainability experienced in the course of the intervention.62 The interviews lasted 30 to 60 minutes and were audiotaped. The REA interview guide (Appendix 9.5) contained 17 semistructured questions (content depending on the informant's role in the facility). When appropriate, answers to questions were followed up with in-depth probes to collect the history of PCTeam implementation from staff most familiar with the IMPACTT project. Interviews included questions on whether the staff thought the PCTeam was successful, how it was structured and operated, if it made a difference in the lives of resident or in the care provided, and what challenges the team faced, with probes requesting specific examples. Field notes were collected based on observations of team and ELNEC training, rounding with the nurse interventionist, stakeholder meetings, and site visits. Written material included email communications between project and facility staff as well as site-developed policies used to formally embed a PCTeam in the facility or brochures marketing this as a service to the public. Interview participants were administrators (n = 7), directors of nursing (n = 9), registered and licensed practical nurses (n = 11), social workers (n = 11), and nursing assistants (n = 3).
Time Frame and Analytical and Statistical Approaches
Defining the Intervention Periods
Resident decedents were assigned to the preintervention period if most of their last 90 days of life occurred before the beginning of the intervention period; otherwise, these decedents were included in the intervention period.
We defined the intervention period in 2 ways. Definition 1 included both the active intervention period (2 months during which the PCTeams actively rounded with the nurse interventionist) and the passive period (the following 8-10 months during which the nurse interventionist could be consulted but did not actively round with the team). Definition 2 included only the passive period. The rationale for employing these 2 definitions is that during the active intervention, NHs were still being coached by the nurse interventionist and that staff's knowledge of palliative and EOL practices was still forming and normalizing during this period. During this period, practices likely had been changing, and hence the intervention might just have begun to have an impact. The passive period (definition 2) reflects on the work of teams that had begun to operate largely without continuing input from the interventionist.
For definition 2, we excluded residents with most of their last 90 days of life falling within the active period because of the ambiguity in their classification.
Because the intervention was rolled out in the 14 treatment facilities on a staggered schedule, no well-defined absolute predates and postdates could apply to the 2 control groups (the randomized control group of 11 NHs and all other facilities in NYS). To handle this analytically, we used Monte Carlo techniques to randomly match control facilities with preperiods and postperiods. We defined preintervention and intervention periods for the usual care and NYS facilities by randomly assigning beginning and ending dates of active and passive periods from one of the intervention facilities. The randomization was repeated and each model estimated 200 times for each of the 2 definitions of the intervention period. We tested the effects of the intervention, both prechanges and postchanges in quality and DIDs, with respect to the unidirectional hypothesis that the intervention improved quality, with P ≤ .05 significance level. We collected coefficients and P values for each replication. We report the average odds ratios (ORs) or average incidence rate ratios (IRRs) and the percentage of each of the 200 model iterations in which the P values for the relevant coefficient were statistically significant (P < .05). The higher this percentage, the greater our confidence that the observed effect is statistically significant.
DID Model
We determined the effect of the intervention by comparing the preintervention and intervention periods and DID—namely, comparing the performance differential between the intervention facilities before and during the intervention with that of the control groups preintervention and during intervention performance differential. We estimated 4 separate models, 1 for each outcome. These resident-level models predict outcomes controlling for the individuals' clinical risk factors, the type of facility in which they resided (eg, intervention or control), and the period (eg, preintervention). We estimated logistic models for the outcomes that were binary (death in the hospital, pain, and depression). For the hospitalization outcome, which was a count variable, we estimated a Poisson model. Specifically, we estimated models of the following general form:
Yij = αj + β1IVi,j + β2UCi,j + γPi,j + δ1IVi,j × Pij + δ2U Cij × Pij + θRFij + Ui,j, where Yij is the outcome (in the case of hospitalization) or its logit (for the other 3 outcomes) for resident i in facility j; IVij is an indicator variable obtaining the value 1 if patient i resided in intervention facility; and UCi,i is an indicator variable obtaining the value 1 if patient i resided in a control facility, with the nonrandomized other NYS facilities serving as the reference. Pij indicates if patient i resided in facility j during the intervention period. The preintervention period served as the reference. The facility type variables (ie, intervention or control) were interacted with the intervention period variable to allow for preintervention and intervention period comparisons for each type of facility. RFij is the vector of risk factors specific to each outcome.53 We estimated the models with facility random effects to allow for clustering of residents within facilities.
Sensitivity Analyses
As originally proposed in our funding application, an integral part of our study design was its use of REAs to better understand barriers/challenges to the intervention and its uptake or sustainability that may have existed in the treatment facilities, how those were dealt with by the NH leadership and staff, and whether the facility's staff thought that their planned and hoped-for PCTeam was fully able to launch and maintain its work. Analysis of in-depth interviews with staff in the treatment homes revealed that only 6 of the 14 facilities had achieved consistently working PCTeams throughout the study period. These teams, in contrast with teams in the other 8 treatment NHs, were characterized by a clear and shared mission, a sense that the team influenced residents' care, and a perception of continued team sustainability. They also appeared to have a more tangible support from and involvement of their facility leaders, including nursing directors and administrators.62 For ease of exposition we refer to these 6 NHs as having “working,” and the other 8 NHs as having “nonworking,” PCTeams. We performed sensitivity analyses, employing the DID models described above, to compare NHs with working and nonworking teams with each other, and with the randomized and nonrandomized controls, on all outcomes of interest.
We also employed a generalized linear model with facility random effects to examine the differences between homes with working and nonworking teams in 5 care process measures (team cohesion, communication/coordination, perceived team performance, perceived PC competency, and organizational readiness for PC), and we performed a DID comparing the 2 types of NHs, preintervention and postintervention.
Conduct of the Study and Changes
Over the study, several noteworthy changes occurred. First, 6 NHs withdrew, reducing the number of facilities to 25 (14 treatment and 11 controls). One additional facility closed. Power calculations on this reduced sample showed the remaining powers of 77% to 85%, for measures of pain and in-hospital deaths, to be still acceptable,54 thus allowing us to test hypothesis 1 on 4 out of 5 proposed outcome measures.
Second, the return rate for the staff surveys in the postintervention wave was well below expectation (466 completed surveys, 21% response rate, from 13 of the 25 NHs). We maintained frequent contact with all facilities, repeatedly communicating with facility leaders to stress the need for higher staff response rates. Four of the 25 NHs did not participate in the second survey wave, citing union/legal issues, changes in ownership, and other competing priorities. We offered raffle prizes to staff to complete the surveys and offered monetary awards to NHs to help us secure a minimal number of responses (30-50, depending on facility size). We offered to visit and hold “survey parties” to encourage greater staff participation. While these efforts did result in some increase in the response rate, it was far from expected or sufficient for analytical purposes; therefore, we were unable to test hypothesis 2 regarding changes in care processes.
Similarly, the return rate for the postintervention bereavement surveys of decedents' families was also well below the expected (14 completed surveys from 14 of the 25 NHs). We had very little control over facilities' willingness to mail these surveys to families, although monetary incentives were offered to all facilities. We were not able to test the impact of the intervention on family caregivers' satisfaction with care (hypothesis 3).
Third, the collection of facility-level data on use of advance directives (ADs) was completed only for the period before the implementation of the intervention. Most facilities in the areas outside of Rochester had substantial gaps in the availability of these data. Furthermore, we found that facilities were overburdened with the data collection requirements (between staff surveys, family surveys, and providing access to ADs to our data collectors). This burden was felt particularly acutely in the treatment NHs. To avoid losing additional facilities to attrition, we made a choice to focus our efforts on postintervention surveys rather than to also continue with postintervention AD collection. This decision was further reinforced by the relative incompleteness of the AD data and the fact that many ADs were transferred to the NH from a hospital rather than completed on site, thus reflecting on the care processes of the hospital, not those of the NH. However, this decision precluded us from including ADs in our outcome analysis.
Fourth, in 5 of the 14 NHs, we shortened the time for passive intervention phase from 10 to 8 months after the active intervention phase of 2 months was completed. This change was necessary to accommodate delayed take up of the intervention, thus delaying the intervention phase. The shortened passive phase allowed these 5 facilities to complete the intervention and be included in the outcome analyses. In the analytical approach, we controlled for the length of the exposure to passive intervention.
Results
Figure 2 presents a CONSORT participant flow diagram depicting study population enrollment. All freestanding NHs in the metropolitan areas of Syracuse, Rochester, and Buffalo were approached for recruitment (n = 136). This included facilities in both urban and rural areas. Based on power calculations, a sample size of 30 facilities was sufficient for the RCT.
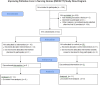
Figure 2
CONSORT Flow Diagram.
The intervention commenced in 2013 with 32 NYS NHs being randomized into treatment (n = 16) and control (n = 16) arms. Facilities were randomly allocated into either arm using a computer-generated, random number–producing algorithm. Early in the study, 7 NHs were lost to follow-up. Five NHs belonging to 1 chain were administratively withdrawn all at once at a corporate level (2 intervention sites and 3 control sites), with administrators citing corporate inconsistency with the study principles, and an additional control NH closed. At the start of the second wave of staff surveys, an additional control NH declined to participate, citing increased workload inconsistent with disseminating the staff survey. The intervention ended in April 2016 with 25 facilities (14 treatment and 11 controls). In analyzing the impact of the intervention, we also included the comparison with all other NYS facilities not involved in the intervention. Using this additional “nonrandomized control” group allows us to test the possibility that the participating control homes may have improved care simply because they were aware of the intervention even though they did not receive it.
Facility-Based Qualitative Results
Through iterative analysis of the REA data, based on focused interviews with 41 staff members from the 14 intervention homes, and from field notes and supplementary written materials from the project coordinator, the TeamSTEPPS trainer, and the ELNEC trainer and nurse-interventionist, we identified key structural themes that influenced facilities' ability to launch and sustain a working team. We briefly report on these findings here because they provided important input into the sensitivity analyses we conducted, as described below. A detailed analysis of the REA data are presented in a manuscript submitted for publication.62
In our analysis of the REA data we identified 5 structural themes: presence or lack of administrative support for the PCTeam; overall financial considerations of the facility; turnover; staffing; and the extent to which there were competing priorities. Although the sustainability of the nascent PCTeams was constantly threatened by competing priorities, we found that the main factor in whether an NH could maintain a working team was consistent and tangible administrative leadership support—something 6 facilities benefited from and 8 facilities lacked. Teams that felt tangibly supported were able to plan for the continued conduct of their work and make the PCTeam part of standard care delivery in their facility. For the most part, these homes successfully tailored their PCTeam to align with their existing care structures. While these teams also appeared to report considerable facility-level turnover, the turnover of their top administrative staff may have been slower and staffing levels in these facilities did not seem to reach a crisis mode, unlike in the facilities that were less able to sustain their PCTeams.
Furthermore, financial considerations were often brought up when discussing PCTeam implementation. Staff members from all NHs were acutely aware of limited financial resources in their organizations and how the development of PCTeams could easily be hindered by financial concerns.
Primary Outcomes
In this section, we focus on testing our primary hypothesis that residents in NHs with PCTeams achieve better EOL risk-adjusted outcomes compared with residents in facilities providing standard care. At baseline, 20.3% of the decedents from treatment NHs were dying in hospitals, compared with 14.8% from the control facilities and 31.1% from all other NYS NHs (Table S.1), and these differences were statistically significantly different (P < .0001). For measures of self-reported pain and depression, decedents in treatment and control homes did not report statistically significantly different symptoms. In the treatment homes, 12.7% and 12.9% of decedents reported moderate-to-severe pain and depressive symptoms, respectively, compared with 11.8% and 11.3%, respectively, in the control NHs. Compared with those in treatment homes, decedents in all other NYS facilities had lower baseline self-reported pain (8.1%; P < .0001), but higher depressive symptoms (15.8%; P < .0001). Preintervention hospitalizations in the last 90 days of life were more frequent in the treatment homes (0.34) compared with control (0.29, P < .05), and less frequent compared with all other NHs (0.44, P < .0001).
In Table 2, treatment and control homes are compared at baseline on several characteristics, including the 4 outcomes of interest. The control homes were not statistically significantly different from the treatment homes on any of the outcome measures, although there were some statistically significant differences between the randomized controls and all other NYS homes (death in hospital and pain), and between treatment and all other NHs (death in hospital). There were no other statistically significant differences at baseline between the treatment and the control NHs, or vis-à-vis the remaining NYS facilities. The comparisons of resident characteristics and risk factors are presented in Supplemental Tables S.1 and S.2, and include all of the risk factors used in building the outcome models.53
Table 2
Baseline Facility Characteristics: RCT Participating and Other Nonrandomized NHs in NYS.
Tables 3 and 4 present the results of the multivariate analyses. The first set of columns show the pre-post differences in each outcome for each group of facilities in terms of the average IRRs for number of hospitalizations and ORs for the other outcomes. The second set of columns, reporting the DID, presents the average of the ratios of ORs or IRRs. If this ratio is <1, residents in the reference facility type performed better relative to the comparison facility.
Table 3
Impact of Intervention on EOL Outcome Measures: Average Odds/Incidence Rate Ratios by RCT Arm.
Table 4
Sensitivity Analysis—Impact of Intervention on EOL Outcomes: Average Odds/Incidence Rate Ratios by RCT Arm and Treatment Facility Type.
Table 3 presents the results of the main multivariate analysis comparing all treatment NHs (working and nonworking groups) with control NHs. When testing the hypothesis of improvement in quality in the postperiod, we found that the large group of nonrandomized NYS facilities showed a significant improvement for pain and depression, but not for the 2 hospitalization measures. The randomized treatment and control groups show no significant improvement, as inferred by examining the percentage of iterations in which the relevant coefficient reached the significance level of 0.05. The impact of the intervention is demonstrated by the DID analysis. We found no significant effects of the intervention, using the same criteria.
Table 4 presents the multivariate analysis comparing treatment NHs with working and nonworking teams, and with the controls. Facilities with working PCTeams exhibited a decline for in-hospital deaths and for depressive symptoms when comparing the preintervention and postintervention periods, while facilities with nonworking teams did not. The second set of columns, reporting the DID results, presents the average of the ratios of ORs or IRRs. If this ratio is <1, the residents in the reference facility type performed better relative to the comparison facility (see Table 4, footnote 4). The DID analysis shows that in facilities with working PCTeams, compared with those facilities with nonworking teams, decedents had improved outcomes for in-hospital death (ratio of around 0.4) and depressive symptoms (ratio of around 0.2). Regarding randomized controls, NHs with working teams improved for in-hospital death (ratio of around 0.5) but only when using the passive intervention period definition. These facilities also improved on this outcome compared with all other NYS facilities with a ratio of about 0.6; however, the DID analysis did not demonstrate a statistically significant difference in depressive symptoms when comparing NHs with working teams and the controls (randomized and nonrandomized).
Secondary Outcomes
Our ability to analyze secondary outcomes and to understand how the intervention may have affected staff care processes and caregivers' satisfaction with care was severely compromised by our inability to secure adequate response rates to the postintervention wave of surveys.
Although we were not able to conduct a DID analysis comparing treatment and control homes on care processes, we had enough data to conduct this analysis on treatment homes known to have working PCTeams and those where PCTeams were not as well functioning throughout the intervention. This analysis allowed us to shed some light on the findings reported in Table 4. In terms of communication/coordination, perceived team effectiveness, and organizational readiness for PC, homes with working teams were significantly better than those with nonworking teams both before and after the intervention; there was no difference related to perceived PC competency (Table 5). The largest pre-post period impact was in communication/coordination (0.078) and the smallest was in PC competency (−0.015). However, the DID analysis showed no statistically significant differences between NHs with working and nonworking teams in any care process measures.
Table 5
Comparison of Mean Differences in Care Processes in Treatment Facilities With and Without Working PCTeams.
Discussion
Study Results in Context
PC intervention research in NHs, particularly using RCT design, has been quite rare. Of the published RCT interventions, several had very circumscribed PC-related objectives, such as improving pain management,63 increasing completion of ADs,64,65 or testing the effect of clinical pathways for pneumonia treatment.66 There have been no RCTs in which PC is viewed as a system of care designed to incorporate all of these components.57 In particular, the effectiveness of facility-based PCTeams on residents' outcomes has not been rigorously evaluated through RCTs.
In this paper, we report the results of a multifaceted RCT designed to create a model of PC delivery in NHs through focused interdisciplinary teams. Our findings are both disappointing and intriguing: disappointing, because we did not demonstrate a significant impact of the intervention on residents' risk-adjusted outcomes, when treating the findings in the traditional intent-to-treat RCT framework; intriguing, because in the sensitivity analysis, in which we bring to bear additional information based on qualitative data, regarding the success of the intervention in developing and sustaining PCTeams, we did find statistically significant impact of the intervention on selected outcomes. Furthermore, our findings from staff surveys revealed that facilities with continuously working PCTeams were significantly more prepared at baseline to incorporate PC into their daily practice, compared with the remaining treatment homes. Compared with the latter, staff in NHs with working teams did not report higher perceived PC skill levels but did report higher (better) scores on all domains of teamwork and on organizational readiness to adopt PC into daily practice. These higher scores continued postintervention, demonstrating greater gains in NHs with working PCTeams compared with those without, regarding communication and coordination and team cohesion. NHs with working PCTeams not only started from a better position but also seem to have been better able to learn the lessons offered by the intervention.
Surprisingly, however, while our outcome analysis detected a significant difference between facilities with and without working PCTeams, the analysis of care processes did not detect statistically significant differences; rather, it demonstrated an effect in the expected direction. It is possible that the outcome measures we used are more sensitive to change than the care process measures so that for the former we were able to detect the impact of the intervention but not for the latter. Another possibility is that the care processes had larger measurement errors that overwhelmed the small effect size of the intervention. Due to high staff turnover, the preintervention and postintervention assessments of care processes were most likely completed by different individuals. While staff responding to preintervention and postintervention surveys in NHs with working teams may have perceived care processes to be good, they most likely did not share the same reference point and thus their responses did not reflect improvement.
Furthermore, it may be argued that an outcome such as death occurring in an NH (as opposed to in-hospital) would indeed be most responsive to improvement in facilities where communication between staff and with residents/families is better, allowing staff to be more familiar with residents' treatment preferences and making sure both residents and their families understand the benefits and drawbacks of hospitalizing patients with advanced illness.11 Better communication among staff is also thought to be a necessary precondition for identifying residents with depression and to improve depression management.67,68 But in order to improve an outcome such as pain, improvements in PC competency, which we did not observe, may be also necessary. We also did not observe a significant effect on the number of hospitalizations occurring in the last 90 days of life. This should not be too surprising, as our intervention did not provide NHs with skills or resources to more effectively manage acute care conditions on site. Good communication alone is likely not enough to reduce hospital transfers; this is consistent with the findings of a recently completed evaluation of the 2012 CMS' Initiative to Reduce Avoidable Hospitalizations in selected facilities in 7 states.69 Only facilities in which advanced practice nurses were well integrated to provide clinical support were able to effect reductions in hospital admissions.
Generalizability of the Findings
Our findings were confined to facilities in 1 region of upper NYS, and while we demonstrated some significant effects of the intervention on resident risk-adjusted outcomes, we are unable to generalize these findings elsewhere.
Implementation of Study Results: Decisional Context
Our findings may be useful for policymakers (eg, CMS) to consider including EOL care measures in the existing repertoire of quality metrics regularly reported on the Medicare.gov Nursing Home Compare website. None of the currently reported quality measures focus on residents at the EOL. Including such measures may incentivize NH administrators to more thoughtfully consider the importance of these outcomes and may prompt family members to make different NH choice decisions. Our findings may also serve to remind policymakers (eg, federal and state government agencies) that NHs, which are notoriously strapped for resources, will, in the absence of regulatory and reimbursement incentives to the contrary, attempt to maximize service delivery to the most lucrative (ie, postacute) patients often to the disadvantage of the less profitable but perhaps more vulnerable long-term residents.
Subpopulation Considerations
Our analysis of the effectiveness of PCTeams in NHs applies only to NH residents who ultimately died during the postintervention retrospective time frame. We did not plan to, and therefore were not able to, evaluate whether other NH residents who were discharged from the facility during the study period, including short-term or rehabilitation-stay residents, as well as all other residents who were still alive after April 16, 2016, benefited from the launch of PCTeams in their respective care facilities. Whether PCTeams had any impact on their care, their hospitalization risk, or on pain or depression symptoms is not known; however, it is reasonable to suggest that dying patients should have received the greatest impact from an intervention designed to improve EOL care and therefore were the only subpopulation chosen for evaluation.
Study Limitations
Several limitations should be noted. First, we are unable to distinguish the relative importance of the individual intervention components. We cannot differentiate if and to what extent the educational efforts—that is, ELNEC and TeamSTEPPS training, coaching by the nurse-interventionist during the active phase or over the duration of the intervention—were effective in stimulating the formation of the PCTeams. However, given that only those homes that were better equipped at baseline to move forward with the intervention were more successful, it may be more important to first understand what made these facilities better to begin with. Second, some treatment homes were clearly better prepared to provide PC than others; however, we were unable to differentiate between these 2 groups of NHs by simply relying on the characteristics available to us for a baseline comparison, before randomization into treatment or control occurred. Thus, it is conceivable that our randomization was not able to control for all possible factors, including readiness to launch and sustain a PCTeam. Third, because we were not successful in obtaining sufficiently large number of responses to postintervention staff surveys and to surveys of families of decedent NH residents, we were not able to test hypotheses 2 and 3. Also due to difficulties in collecting information on ADs from the participating NHs, our analyses relating to hypothesis 1 (measures of outcomes) had to omit this metric. These experiences suggest that future interventions in NHs may need to either minimize data collections (possibly narrowing the scope of the intervention) that require the effort/cooperation of NH staff or, alternatively, consider devoting a substantial amount of funding to pay NHs for their participation and data collection—yet, at the same time, ensuring that no undue biases are introduced in the process.
Future Research
This project provides evidence that it is possible to implement sustainable facility-based PCTeams without necessarily employing expensive outside full-time PC staff during the intervention period, as other models have proposed (eg, in CMS demonstration projects).70 However, future research may need to focus on the baseline facility-based factors and characteristics that permitted some NHs to sustain this intervention while others, equally receptive to the concept of launching a PCTeam, fell short of independently supporting this model.
Conclusions
Increasing PC capacity in NHs has been deemed essential to the provision of high-quality care for residents with chronic illness and those nearing the EOL. And while the experts' voices endorsing PC in this setting have been increasing, evidence on the scope, depth, or effectiveness of PC in NHs has been very scant, and reimbursable models for PC delivery, outside of hospice, are largely nonexistent in NHs. In fact, current reimbursement policy incentives and business models favor the delivery of postacute care and do not incentivize NHs to focus on PC provision for their residents.
In this context, we conducted an RCT implementing PCTeams in NHs to improve residents' EOL outcomes. Prior studies have suggested that improving palliative and EOL care in NHs is much needed. Our study demonstrates that interventions may provide NHs that at baseline are primed to implement PC with an opportunity to succeed, but interventions alone are not likely to effect broad and generalizable improvements. Policy changes that prioritize and incentivize facilities to adopt palliative and EOL care practices, and regulatory efforts to include performance measures that are specific to patients with advanced illness, are needed to create an environment in which effective PC can become sustainable.
References
- 1.
- Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. National Academies Press; 2014. [PubMed: 25927121]
- 2.
- Weitzen S, Teno JM, Fennell M, Mor V. Factors associated with site of death: a national study of where people die. Med Care. 2003;41(2):323-335. doi:10.1097/01.MLR.0000044913.37084.27 [PubMed: 12555059] [CrossRef]
- 3.
- Gruneir A, Mor V. Nursing home safety: current issues and barriers to improvement. Annu Rev Public Health. 2008;29:369-382. doi:10.1146/annurev.publhealth.29.020907.090912 [PubMed: 18173385] [CrossRef]
- 4.
- Temkin-Greener H, Zheng N, Norton SA, Quill T, Ladwig S, Veazie P. Measuring end-of-life care processes in nursing homes. Gerontologist. 2009;49(6):805-813. doi:10.1093/geront/gnp092 [PMC free article: PMC2775900] [PubMed: 19574538] [CrossRef]
- 5.
- Unroe KT, Cagle JG, Lane KA, Callahan CM, Miller SC. Nursing home staff palliative care knowledge and practices: results of a large survey of frontline workers. J Pain Symptom Manage. 2015;50(5):622-629. doi:10.1016/j.jpainsymman.2015.06.006 [PMC free article: PMC4755479] [PubMed: 26150325] [CrossRef]
- 6.
- Teno JM, Kabumoto G, Wetle T, Roy J, Mor V. Daily pain that was excruciating at some time in the previous week: prevalence, characteristics, and outcomes in nursing home residents. J Am Geriatr Soc. 2004;52(5):762-767. doi:10.1111/j.1532-5415.2004.52215.x [PubMed: 15086658] [CrossRef]
- 7.
- Hanson LC, Eckert JK, Dobbs D, et al. Symptom experience of dying long-term care residents. J Am Geriatr Soc. 2008;56(1):91-98. doi:10.1111/j.1532-5415.2007.01388.x [PubMed: 17727647] [CrossRef]
- 8.
- Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212-1221. doi:10.1056/NEJMsa1100347 [PMC free article: PMC3236369] [PubMed: 21991894] [CrossRef]
- 9.
- Xing J, Mukamel DBDB, Temkin-Greener H. Hospitalizations of nursing home residents in the last year of life: nursing home characteristics and variation in potentially avoidable hospitalizations. J Am Geriatr Soc. 2013;61(11):1900-1908. doi:10.1111/jgs.12517 [PMC free article: PMC3827689] [PubMed: 24219191] [CrossRef]
- 10.
- Zheng NT, Temkin-Greener H. End-of-life care in nursing homes: the importance of CNA staff communication. J Am Med Dir Assoc. 2010;11(7):494-499. [PMC free article: PMC2935906] [PubMed: 20816337]
- 11.
- Temkin-Greener H, Li Q, Li Y, Segelman M, Mukamel DB. End-of-life care in nursing homes: from care processes to quality. J Palliat Med. 2016;19(12):1304-1311. doi:10.1089/jpm.2016.0093 [PMC free article: PMC5144883] [PubMed: 27529742] [CrossRef]
- 12.
- Temkin-Greener H, Zheng NT, Cai S, Zhao H, Mukamel DB. Nursing home environment and organizational performance: association with deficiency citations. Med Care. 2010;48(4):357-364. doi:10.1097/MLR.0b013e3181ca3d70 [PMC free article: PMC2925404] [PubMed: 20220535] [CrossRef]
- 13.
- Whittaker E, George Kernohan W, Hasson F, Howard V, McLaughlin D. The palliative care education needs of nursing home staff. Nurse Educ Today. 2006;26(6):501-510. doi:10.1016/j.nedt.2006.01.004 [PubMed: 16517029] [CrossRef]
- 14.
- Whittaker E, George Kernohan W, Hasson F, Howard V, McLaughlin D. Palliative care in nursing homes: exploring care assistants' knowledge. Int J Older People Nurs. 2007;2(1):36-44. doi:10.1111/j.1748-3743.2007.00038.x [PubMed: 20925830] [CrossRef]
- 15.
- Gittell J, Weinberg D, Pfefferle S, Bishop C. Impact of relational coordination on job satisfaction and quality outcomes: a study of nursing homes. Hum Resour Manage. 2008;18(2):154-170. doi:10.1111/j.1748-8583.2007.00063.x [CrossRef]
- 16.
- Temkin-Greener H, Cai S, Zheng NT, Zhao H, Mukamel DB. Nursing home work environment and the risk of pressure ulcers and incontinence. Health Serv Res. 2012;47:1179-1200. doi:10.1111/j.1475-6773.2011.01353.x [PMC free article: PMC3290703] [PubMed: 22098384] [CrossRef]
- 17.
- Miller SC, Lima JC, Thompson SA. End-of-life care in nursing homes with greater versus less palliative care knowledge and practice. J Palliat Med. 2015;18(6):527-534. doi:10.1089/jpm.2014.0393 [PMC free article: PMC4519050] [PubMed: 25774449] [CrossRef]
- 18.
- Hanson LC, Ersek M. Meeting palliative care needs in post-acute care settings. JAMA. 2006;295(6):681-686. doi:10.1001/jama.295.6.681 [PubMed: 16467237] [CrossRef]
- 19.
- Gozalo PL, Miller SC. Hospice enrollment and evaluation of its causal effect on hospitalization of dying nursing home patients. Health Serv Res. 2007;42(2):587-610. doi:10.1111/j.1475-6773.2006.00623.x [PMC free article: PMC1955355] [PubMed: 17362208] [CrossRef]
- 20.
- Miller SC, Mor V, Wu N, Gozalo P, Lapane K. Does receipt of hospice care in nursing homes improve the management of pain at the end of life? J Am Geriatr Soc. 2002;50(3):507-515. doi:10.1046/j.1532-5415.2002.50118.x [PubMed: 11943048] [CrossRef]
- 21.
- Temkin-Greener H, Zheng NT, Xing J, Mukamel DB. Site of death among nursing home residents in the United States: changing patterns, 2003-2007. J Am Med Dir Assoc. 2013;14(10):741-748. doi:10.1016/j.jamda.2013.03.009 [PMC free article: PMC3744590] [PubMed: 23664483] [CrossRef]
- 22.
- Parker-Oliver D, Bickel D. Nursing home experience with hospice. J Am Med Dir Assoc. 2002;3(2):46-50. doi:10.1016/S1525-8610(04)70412-0 [PubMed: 12807538] [CrossRef]
- 23.
- Wetle T, Teno JM, Shield R, Welch LC, Miller SC. End of Life in Nursing Homes: Experiences and Policy Recommendations. American Association of Retired Persons (AARP); 2004.
- 24.
- Miller SC, Lima J, Gozalo PL, Mor V. The growth of hospice care in U.S. nursing homes. J Am Geriatr Soc. 2010;58(8):1481-1488. doi:10.1111/j.1532-5415.2010.02968.x [PMC free article: PMC2955193] [PubMed: 20646101] [CrossRef]
- 25.
- Huskamp HA, Stevenson DG, Grabowski DC, Brennan E, Keating NL. Long and short hospice stays among nursing home residents at the end of life. J Palliat Med. 2010;13(8):957-964. doi:10.1089/jpm.2009.0387 [PubMed: 20666661] [CrossRef]
- 26.
- Kovach C, Volicer L, Hurley A. Effects of hospice intervention in nursing home residents with later stages of dementia. In: Hospice Care for Patients With Advanced Progressive Dementia. Volicer L, Hurley A, eds. Springer; 1998:276-292.
- 27.
- Hanson LC, Reynolds KS, Henderson M, Pickard CG. A quality improvement intervention to increase palliative care in nursing homes. J Palliat Med. 2005;8(3):576-584. doi:10.1089/jpm.2005.8.576 [PubMed: 15992199] [CrossRef]
- 28.
- Higginson IJ, Finlay IG, Goodwin DM, et al. Is there evidence that palliative care teams alter end-of-life experiences of patients and their caregivers? J Pain Symptom Manage. 2003;25(2):150-168. doi:10.1016/S0885-3924(02)00599-7 [PubMed: 12590031] [CrossRef]
- 29.
- Gelfman LP, Meier DE, Morrison RS. Does palliative care improve quality? a survey of bereaved family members. J Pain Symptom Manage. 2008;36(1):22-28. doi:10.1016/j.jpainsymman.2007.09.008 [PMC free article: PMC2527760] [PubMed: 18411019] [CrossRef]
- 30.
- May P, Normand C, Morrison RS. Economic impact of hospital inpatient palliative care consultation: Review of current evidence and directions for future research. J Palliative Med. 2014;17(9), 1054-1063. doi:10.1089/jpm.2013.0594. [PMC free article: PMC4158953] [PubMed: 24984168] [CrossRef]
- 31.
- Laguna J, Goldstein R, Allen J, Braun W, Enguídanos S. Inpatient palliative care and patient pain: pre- and post-outcomes. J Pain Symptom Manage. 2012;43(6):1051-1059. doi:10.1016/j.jpainsymman.2011.06.023 [PubMed: 22651948] [CrossRef]
- 32.
- Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190. doi:10.1089/jpm.2007.0055 [PubMed: 18333732] [CrossRef]
- 33.
- Ranganathan A, Dougherty M, Waite D, Casarett D. Can palliative home care reduce 30-day readmissions? results of a propensity score matched cohort study. J Palliat Med. 2013;16(10):1290-1293. doi:10.1089/jpm.2013.0213 [PMC free article: PMC3791031] [PubMed: 24007348] [CrossRef]
- 34.
- Amano K, Morita T, Tatara R, Katayama H, Uno T, Takagi I. Association between early palliative care referrals, inpatient hospice utilization, and aggressiveness of care at the end of life. J Palliat Med. 2015;18(3):270-273. doi:10.1089/jpm.2014.0132 [PubMed: 25210851] [CrossRef]
- 35.
- Brody AA, Ciemins E, Newman J, Harrington C. The effects of an inpatient palliative care team on discharge disposition. J Palliat Med. 2010;13(5):541-548. doi:10.1089/jpm.2009.0300 [PubMed: 20210658] [CrossRef]
- 36.
- Tangeman JC, Rudra CB, Kerr CW, Grant PC. A hospice-hospital partnership: reducing hospitalization costs and 30-day readmissions among seriously ill adults. J Palliat Med. 2014;17(9):1005-1010. doi:10.1089/jpm.2013.0612 [PubMed: 24921158] [CrossRef]
- 37.
- Fromme EK, Bascom PB, Smith MD, et al. Survival, mortality, and location of death for patients seen by a hospital-based palliative care team. J Palliat Med. 2006;9(4):903-911. doi:10.1089/jpm.2006.9.903 [PubMed: 16910805] [CrossRef]
- 38.
- Temel J, Greer J, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363(8):733-742. doi:10.1056/NEJMoa1000678 [PubMed: 20818875] [CrossRef]
- 39.
- Huskamp HA, Stevenson DG, Chernew ME, Newhouse JP. A new Medicare end-of-life benefit for nursing home residents. Health Aff (Millwood). 2010;29(1):130-135. doi:10.1377/hlthaff.2009.0523 [PubMed: 20048371] [CrossRef]
- 40.
- Temkin-Greener H, Ladwig S, Caprio T, et al. Developing palliative care practice guidelines and standards for nursing home–based palliative care teams: a Delphi study. J Am Med Dir Assoc. 2015;16(1):86.e1-e7. doi:10.1016/j.jamda.2014.10.013 [PubMed: 25481748] [CrossRef]
- 41.
- Froggatt K, Wilson D, Justice C, et al. End-of-life care in long-term care settings for older people: a literature review. Int J Older People Nurs. 2006;1(1):45-50. doi:10.1111/j.1748-3743.2006.00008.x [PubMed: 20925728] [CrossRef]
- 42.
- Ersek M, Grant MM, Kraybill BM. Enhancing end-of-life care in nursing homes: Palliative Care Educational Resource Team (PERT) program. J Palliat Med. 2005;8(3):556-566. doi:10.1089/jpm.2005.8.556 [PubMed: 15992197] [CrossRef]
- 43.
- Keay TJ, Alexander C, McNally K, Crusse E, Eger RE. Nursing home physician educational intervention improves end-of-life outcomes. J Palliat Med. 2003;6(2):205-213. doi:10.1089/109662103764978452 [PubMed: 12854937] [CrossRef]
- 44.
- Baier RR, Gifford DR, Patry G, et al. Ameliorating pain in nursing homes: a collaborative quality-improvement project. J Am Geriatr Soc. 2004;52(12):1988-1995. doi:10.1111/j.1532-5415.2004.52553.x [PubMed: 15571532] [CrossRef]
- 45.
- Rantz MJ, Popejoy L, Petroski GF, et al. Randomized clinical trial of a quality improvement intervention in nursing homes. Gerontologist. 2001;41(4):525-538. doi:10.1093/geront/41.4.525 [PubMed: 11490051] [CrossRef]
- 46.
- Ltd B. Do evidence-based guidelines improve the quality of care? Evidence-Based Healthc Public Heal. 2005;9(4):270-275. doi:10.1016/j.ehbc.2005.05.001 [CrossRef]
- 47.
- Farmer AP, Legare F, Turcot L, et al. Printed educational materials: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2008;(3):CD004398. doi:10.1002/14651858.CD004398.pub2 [PubMed: 18646106] [CrossRef]
- 48.
- Olsan T, Ladwig S, Temkin-Greener H. Advancing Palliative Care in Nursing Homes: What Matters Most to Residents and Family Members? 2017. Unpublished.
- 49.
- Rycroft-Malone J. Theory and knowledge translation. Nurs Res. 2007;56(4):S78-S85. doi:10.1097/01.NNR.0000280631.48407.9b [PubMed: 17625479] [CrossRef]
- 50.
- Argote L. Organizational Learning: Creating, Retaining and Transferring Knowledge. Springer; 2013. doi:10.1007/978-1-4614-5251-5 [CrossRef]
- 51.
- Hays JC, Galanos AN, Palmer TA, McQuoid DR, Flint EP. Preference for place of death in a continuing care retirement community. Gerontologist. 2001;41(1):123-128. doi:10.1093/geront/41.1.123 [PubMed: 11220809] [CrossRef]
- 52.
- Mukamel DB, Caprio T, Ahn R, et al. End-of-life quality-of-care measures for nursing homes: place of death and hospice. J Palliat Med. 2012;15(4):438-446. doi:10.1089/jpm.2011.0345 [PMC free article: PMC3322517] [PubMed: 22500481] [CrossRef]
- 53.
- Mukamel DDB, Ladd H, Caprio T, Temkin-Greener H. Prototype end-of-life quality measures based on MDS 3 data. Med Care. 2016;54(11):1024-1032. doi:10.1097/MLR.0000000000000576 [PubMed: 27261636] [CrossRef]
- 54.
- Temkin-Greener H, Ladwig S, Ye Z, Norton SA, Mukamel DB. Improving Palliative Care Through Teamwork (IMPACTT) in nursing homes: study design and baseline findings. Contemp Clin Trials. 2017;56:1-8. [PubMed: 28315478]
- 55.
- Kelly K, Ersek M, Virani R, Malloy P, Ferrell B; End-of-Life Nursing Education Consortium. Geriatric training program: improving palliative care in community geriatric care settings. J Gerontol Nurs. 2008;34(5):28-35. doi:10.3928/00989134-20080501-06 [PubMed: 18512631] [CrossRef]
- 56.
- Kelly K, Thrane S, Virani R, Malloy P, Ferrell B. Expanding palliative care nursing education in California: the ELNEC geriatric project. Int J Palliat Nurs. 2011;17(4):188-194. doi:10.12968/ijpn.2011.17.4.188 [PubMed: 21537321] [CrossRef]
- 57.
- Ersek M, Carpenter JG. Geriatric palliative care in long-term care settings with a focus on nursing homes. J Palliat Med. 2013;16(10):1180-1187. doi:10.1089/jpm.2013.9474 [PMC free article: PMC3996937] [PubMed: 23984636] [CrossRef]
- 58.
- Temkin-Greener H, Gross D, Kunitz SJ, Mukamel D. Measuring interdisciplinary team performance in a long-term care setting. Med Care. 2004;42(5):472-481. doi:10.1097/01.mlr.0000124306.28397.e2 [PubMed: 15083108] [CrossRef]
- 59.
- Temkin-Greener H, Zheng N, Katz P, Zhao H, Mukamel DB. Measuring work environment and performance in nursing homes. Med Care. 2009;47(4): 482-491. doi:10.1097/MLR.0b013e318190cfd3 [PMC free article: PMC2663940] [PubMed: 19330892] [CrossRef]
- 60.
- Teno JM, Okun SN, Casey V, Welch LC. Toolkit of Instruments to Measure End of Life Care (TIME). http://www
.chcr.brown .edu/pcoc/toolkit.htm. - 61.
- Beebe J. Rapid Assessment Process: An Introduction. AltaMira Press; 2001.
- 62.
- Norton SA, Ladwig S, Caprio TV, Quill TE, Temkin-Greener H. Staff experiences forming and sustaining palliative care teams in nursing homes. Gerontologist. 2018;58(4):e218-e225. doi:10.1093/geront/gnx201 [PubMed: 29309584] [CrossRef]
- 63.
- Jones KR, Fink R, Vojir C, et al. Translation research in long-term care: improving pain management in nursing homes. Worldviews Evid Based Nurs. 2004;1(Suppl 1):S13-S20. doi:10.1111/j.1524-475X.2004.04045.x [PubMed: 17129330] [CrossRef]
- 64.
- Molloy DW, Guyatt GH, Goeree R, et al. Systematic implementation of an advance directive program in nursing homes. JAMA. 2000;283(11):1437. doi:10.1001/jama.283.11.1437 [PubMed: 10732933] [CrossRef]
- 65.
- Lindner SA, Ben Davoren J, Vollmer A, Williams B, Seth Landefeld C. An electronic medical record intervention increased nursing home advance directive orders and documentation. J Am Geriatr Soc. 2007;55(7):1001-1006. doi:10.1111/j.1532-5415.2007.01214.x [PubMed: 17608871] [CrossRef]
- 66.
- Loeb M, Carusone SC, Goeree R, et al. Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295(21):2503-2510. doi:10.1001/jama.295.21.2503 [PubMed: 16757722] [CrossRef]
- 67.
- Brown EL, Raue PJ, Klimstra S, Mlodzianowski AE, Greenberg RL, Bruce ML. An intervention to improve nurse-physician communication in depression care. Am J Geriatr Psychiatry. 2010;18(6):483-490. doi:10.1097/JGP.0b013e3181bf9efa [PMC free article: PMC8950104] [PubMed: 21217559] [CrossRef]
- 68.
- Sprangers S, Dijkstra K, Romijn-Luijten A. Communication skills training in a nursing home: effects of a brief intervention on residents and nursing aides. Clin Interv Aging. 2015;10:311-319. doi:10.2147/CIA.S73053 [PMC free article: PMC4309793] [PubMed: 25653513] [CrossRef]
- 69.
- Ingber M, Feng Z, Khatutsky G, et al. Initiative to reduce avoidable hospitalizations among nursing facility residents shows promising results. Health Aff (Millwood). 2017;36(3):441-450. [PubMed: 28264945]
- 70.
- Unroe KT, Nazir A, Holtz LR, et al. The optimizing patient transfers, impacting medical quality, and improving symptoms: transforming institutional care approach; preliminary data from the implementation of a centers for Medicare and Medicaid services nursing facility demonstration project. J Am Geriatr Soc. 2015;63(1):165-169. doi:10.1111/jgs.13141 [PubMed: 25537789] [CrossRef]
Study-Related Peer Reviewed Journal Publications
- •.
- Temkin-Greener H, Ladwig S, Caprio T, et al. Developing palliative care practice guidelines and standards for nursing home–based palliative care teams: a Delphi study. J Am Med Dir Assoc. 2015;16(1):86.e1-e7. doi:10.1016/j.jamda.2014.10.013 [PubMed: 25481748] [CrossRef]
- •.
- Mukamel DB, Ladd H, Caprio T, Temkin-Greener H. Prototype end-of-life quality measures based on MDS 3 data. Med Care. 2016;54(11):1024-1032. doi:10.1097/MLR.0000000000000576 [PubMed: 27261636] [CrossRef]
- •.
- Temkin-Greener H, Li Q, Li Y, Segelman M, Mukamel DB. End-of-life care in nursing homes: from care processes to quality. J Palliat Med. 2016;19(12):1304-1311. doi:10.1089/jpm.2016.0093 [PMC free article: PMC5144883] [PubMed: 27529742] [CrossRef]
- •.
- Temkin-Greener H, Ladwig S, Ye Z, Norton SA, Mukamel DB. Improving palliative care through teamwork (IMPACTT) in nursing homes: study design and baseline findings. Contemp Clin Trials. 2017;56:1-8. [PubMed: 28315478]
- •.
- Temkin-Greener H, Mukamel DB, Ladd H, et al. Impact of nursing home palliative care teams on end-of-life outcomes: a randomized controlled trial. Med Care. 2018;56(1):11-18. doi:10.1097/MLR.0000000000000835 [PubMed: 29068904] [CrossRef]
- •.
- Norton SA, Ladwig S, Caprio TV, Quill TE, Temkin-Greener H. Staff experiences forming and sustaining palliative care teams in nursing homes. Gerontologist. 2018;58(4):e218-e225. doi:10.1093/geront/gnx201 [PubMed: 29309584] [CrossRef]
Acknowledgment
Research reported in this report was [partially] funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (#641) Further information available at: https://www.pcori.org/research-results/2012/do-palliative-care-teams-nursing-homes-improve-quality-end-life-care-nursing
Appendices
Appendix.
Supplemental Tables: Resident Characteristics at Baseline: By Type of Facility (PDF, 312K)
Appendix 9.1.
Information Letter to NIH Staff Introducing Survey, Stamped Approved by IRB (PDF, 161K)
Appendix 9.2.
Staff Survey: “Tell Us About Your Nursing Home” (PDF, 207K)
Appendix 9.3.
Semistructured Interview Guide for Nursing Home Residents (PDF, 133K)
Appendix 9.4.
Semistructured Interview Guide for Family Caregivers (PDF, 86K)
Appendix 9.5.
Rapid Ethnographic Assessment: Semistructured Interview Guide (PDF, 107K)
Appendix 9.6.
Preferred Palliative Care Best Practices for Nursing Homes (PDF, 129K)
Suggested citation:
Temkin-Greener H, Mukamel DB, Ladwig S, et al. (2019). Do Palliative Care Teams in Nursing Homes Improve the Quality of End-of-Life Care for Nursing Home Residents? Patient-Centered Outcomes Research Institute (PCORI)) https://doi.org/10.25302/7.2019.CER.641
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- Do Palliative Care Teams in Nursing Homes Improve the Quality of End-of-Life Car...Do Palliative Care Teams in Nursing Homes Improve the Quality of End-of-Life Care for Nursing Home Residents?
Your browsing activity is empty.
Activity recording is turned off.
See more...
