Sialic acids and other Nonulosonic acids (NulOs)
Reference: Cataloging natural sialic acids and other nonulosonic acids (NulOs), and their representation using the Symbol Nomenclature for Glycans, Glycobiology 33: 99-103, 2023. Citation link (PMID 36648443).
Sialic acids (Sia) are a family of related monosaccharides commonly present in higher animals like Echinoderma, Hemichorda, Cephalocorda, and Vertebrata. Sialic acids commonly occur as terminal and sometimes as internal residues in glycoconjugates and other carbohydrates, e.g. glycoproteins, glycolipids, lipooligo- & capsular-polysaccharides, tissue polysialic acids, oligosaccharides, and exopolysaccharides. Due to their common terminal presentation on cell surface glycans, they facilitate numerous key biological functions related to cellular recognition, cell adhesion, communication/signaling, control of glycoconjugate half-life in circulation, tumor growth and metastasis, developmental programming, immune regulation, and host interaction with viruses and bacteria, including commensals, symbionts, and opportunistic pathogens.
Sialic acids are a subclass of a superfamily called non-2-ulosonic acids, nonulosonic acids (2-ketoaldonic acids; α-ketoaldonic acids) or "NulOs". The simplest compound in this class is neuraminic acid or Neu (Figure 1), a 3-deoxy, 5-amino form of the nine-carbon base nonulosonic acid, with hydroxyl groups at C4, C7, C8 and C9 conforming with the D-glycero-D-galacto configuration. This molecule contains a 2-keto carboxylic acid group that facilitates C2-O-C6 ring closure. The common intramolecular (cyclic) hemiketal form of Neu is a pyranose ring with 2C5 chair conformation and an equatorially oriented glycerol chain at C6. Addition of a substituent N-acetyl at C5 results in Neu5Ac (N-acetylneuraminic acid). Hydroxylation of the N-acetyl group of Neu5Ac results in Neu5Gc (N-glycolylneuraminic acid). Replacement of the C5 amine with hydroxyl results in Kdn. Neu5Ac, Neu5Gc and Kdn are the most common structural relatives of Neu. These deoxy-nonulosonic acids (deoxy at C3, in all cases) can be further modified by various substituents to result in over 90 additional naturally occurring members of the sialic acid family (Table 1 below).
B. 5-Acetamido-3,5-dideoxy-D-glycero-D-galacto-non-2-ulosonic acid (N-acetylneuraminic acid, Neu5Ac);
C. 3,5-Dideoxy-5-hydroxyacetamido-D-glycero-D-galacto-non-2-ulosonic acid (N-glycolylneuraminic acid, Neu5Gc);
D. 3-Deoxy-D-glycero-D-galacto-non-2-ulosonic acid (2-keto-3-deoxy-nononic acid, ketodeoxynononic acid, Kdn).
In addition to the above, another "sialic-acid-like" subclass of the NulO superfamily exists based on modifications of dideoxy-nonulosonic acid (deoxy at C3 and C9, in all cases). These were originally identified in bacterial lipopolysaccharides, capsular polysaccharides and glycoproteins. The parent compound (5,7-diamino-3,5,7,9-tetradeoxy-non-2-ulosonic acid) in this class contains a terminal methyl group as C9 and aminodeoxy functions at C5 and C7. Six further subclasses have been defined including pseudaminic acid (Pse), legionaminic acid (Leg), 4-epi-legionaminic acid (4eLeg), 8-epi-legionaminic acid (8eLeg), acinetaminic acid (Aci) and 8-epi-acinetaminic acid (8eAci) (Figure 2). A survey of over 50 known structures of naturally occurring 5,7-diamino-3,5,7,9-tetradeoxy-non-2-ulosonic acid derivatives, together with some related 9-deoxy-non-2-ulosonic acids, is presented in Table 2.
NulO biosynthetic pathways couple pyruvate with a hexose (e.g. D-mannose), a N-acetylhexosamine (2-acetamido-2-deoxy-hexose, e.g. N-acetyl-D-mannosamine), or a 2,4-diacetamido-2,4,6-trideoxy-hexose. The hexose (variant) C1-C6 chain becomes carbon 4 to 9 in the NulO, while carbon positions 1 to 3 are contributed by pyruvate. According to IUPAC/IUBMB rules, naming of hexose configurations refers to the first four chiral atoms. In NulO biosynthesis, a new chiral atom is created at C4, generating a seeming incongruence between the nomenclature and how these biological molecules come together in nature. For example, the sialic acid Neu5Ac (5-acetamido-3,5-dideoxy-D-glycero-D-galacto-non-2-ulosonic acid) is formed from N-acetyl-D-mannosamine, with the D-manno becoming C5 to C8 without a change in configuration. However, the new chiral atom created at C4 shifts the nomenclature to instead refer to positions C4 to C7, thus adopting a D-galacto configuration. The D-glycero part of the IUPAC name refers to the configuration at C8, changes to which do not impact the ring configuration. Some aspects of NulO biosynthesis are different between prokaryotes and eukaryotes. However, all NulOs share the unique feature (among the monosaccharides) of being activated using cytidine triphosphate (CTP) as an energy source to create the CMP-NulO donor.
B. 5,7-Diamino-3,5,7,9-tetradeoxy-D-glycero-D-galacto-non-2-ulosonic acid (legionaminic acid, Leg);
C. 5,7-Diamino-3,5,7,9-tetradeoxy-D-glycero-D-talo-non-2-ulosonic acid (4-epi-legionaminic acid, 4eLeg);
D. 5,7-Diamino-3,5,7,9-tetradeoxy-L-glycero-D-galacto-non-2-ulosonic acid (8-epi-legionaminic acid, 8eLeg);
E. 5,7-Diamino-3,5,7,9-tetradeoxy-L-glycero-L-altro-non-2-ulosonic acid (acinetaminic acid, Aci);
F. 5,7-Diamino-3,5,7,9-tetradeoxy-D-glycero-L-altro-non-2-ulosonic acid (8-epi-acinetaminic acid, 8eAci).
Table 1 Reported structures of naturally occurring members of the sialic acid family based on NMR and/or mass spectrometry reports.
(updated survey since the publication of R. Schauer & J.P. Kamerling, Exploration of the Sialic Acid World, Adv. Carbohydr. Chem. Biochem. 2018, 75, 1-213; combined with SNFG symbols) (Download TSV data file)
| Name | Abbreviation | Structure | CID | SID | SNFG | References | Database links |
|---|---|---|---|---|---|---|---|
| 5-Amino-3,5-dideoxy-D-glycero-D-galacto-non-2-ulosonic acid / neuraminic acid (Neu) | Neu | 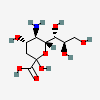 | 441037 | 252301019 | 1, 2, 3, 4, 5, 6 | [CSDB] | |
| Neuraminic acid 1,5-lactamb,i | Neu1,5lactam | 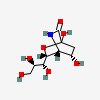 | 168009038 | 480493132 | 6, 7 | ||
| 5-N-Acetyl-neuraminic acid (N-Acetylneuraminic acid) Neu5Ac | Neu5Ac | 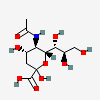 | 439197 | 252089093 | 5, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 | [CSDB1][CSDB2] | |
| 5-N-Acetyl-4-O-acetyl-neuraminic acid | Neu4,5Ac2 |  | 92042742 | 480493133 | 5, 8, 10, 11, 14, 16, 17, 18, 19, 20, 21 | ||
| 5-N-Acetyl-7-O-acetyl-neuraminic acid | Neu5,7Ac2 | 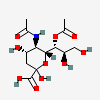 | 92042743 | 480493134 | 5, 8, 10, 11, 16, 17, 18, 19, 20, 22, 23, 24, 25 | ||
| 5-N-Acetyl-8-O-acetyl-neuraminic acid | Neu5,8Ac2 |  | 129677032 | 480493135 | 5, 8, 17, 19, 20, 24 | ||
| 5-N-Acetyl-9-O-acetyl-neuraminic acid | Neu5,9Ac2 | 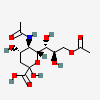 | 92042744 | 480493136 | 5, 8, 14, 16, 17, 18, 19, 20, 23, 24, 25, 26 | ||
| 5-N-Acetyl-4,9-di-O-acetyl-neuraminic acid | Neu4,5,9Ac3 | 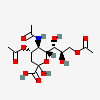 | 129711380 | 480493137 | 5, 8, 14, 17, 20 | ||
| 5-N-Acetyl-7,8-di-O-acetyl-neuraminic acid | Neu5,7,8Ac3 | 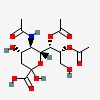 | 168009039 | 480493138 | 22 | ||
| 5-N-Acetyl-7,9-di-O-acetyl-neuraminic acid | Neu5,7,9Ac3 |  | 129711399 | 480493139 | 5, 8, 14, 16, 17, 18, 19, 20, 24, 25, 27 | ||
| 5-N-Acetyl-8,9-di-O-acetyl-neuraminic acid | Neu5,8,9Ac3 | 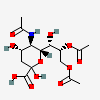 | 129716696 | 480493140 | 5, 8, 16, 17, 18, 19, 20, 24, 25, 27 | ||
| 5-N-Acetyl-4,7,9-tri-O-acetyl-neuraminic acid | Neu4,5,7,9Ac4 |  | 140507949 | 480493141 | 5 | ||
| 5-N-Acetyl-7,8,9-tri-O-acetyl-neuraminic acid | Neu5,7,8,9Ac4 | 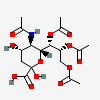 | 129711405 | 480493142 | 8, 17, 18, 19, 20, 24 | ||
| 5-N-Acetyl-4,7,8,9-tetra-O-acetyl-neuraminic acid | Neu4,5,7,8,9Ac5 | 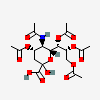 | 11733494 | 480493143 | 5 | ||
| 5-N-Acetyl-4-O-glycolyl-neuraminic acid | Neu5Ac4Gc | 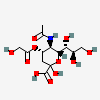 | 168009040 | 480493144 | 28 | ||
| 5-N-Acetyl-7-O-glycolyl-neuraminic acid | Neu5Ac7Gc |  | 168009041 | 480493145 | 28 | ||
| 5-N-Acetyl-9-O-lactyl-neuraminic acidc | Neu5Ac9Lt | 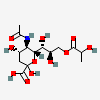 | 129729663 | 480493146 | 5, 8, 17, 19, 20, 29, 30 | ||
| 5-N-Acetyl-4-O-acetyl-9-O-lactyl-neuraminic acid | Neu4,5Ac29Lt | 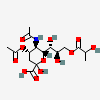 | 129800763 | 480493147 | 5, 8, 17, 20, 31 | ||
| 5-N-Acetyl-7-O-acetyl-9-O-lactyl-neuraminic acid | Neu5,7Ac29Lt | 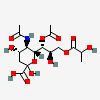 | 140507951 | 480493148 | 5 | ||
| 5-N-Acetyl-8-O-acetyl-9-O-lactyl-neuraminic acid | Neu5,8Ac29Lt | 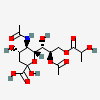 | 168009042 | 480493149 | 32 | ||
| 5-N-Acetyl-8-O-methyl-neuraminic acid | Neu5Ac8Me | 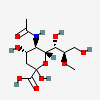 | 14160881 | 480493150 | 5, 8, 12, 20, 33 | ||
| 5-N-Acetyl-4-O-acetyl-8-O-methyl-neuraminic acid | Neu4,5Ac28Me | 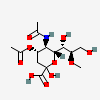 | 168009043 | 480493151 | 5 | ||
| 5-N-Acetyl-9-O-acetyl-8-O-methyl-neuraminic acid | Neu5,9Ac28Me |  | 140507954 | 480493152 | 5, 8, 20, 33 | ||
| 5-N-Acetyl-9-O-methyl-neuraminic acid | Neu5Ac9Me |  | 168009044 | 480493153 | 34 | ||
| 5-N-Acetyl-4-O-sulpho-neuraminic acid | Neu5Ac4S | 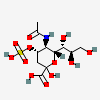 | 168009045 | 480493154 | 35 | ||
| 5-N-Acetyl-8-O-sulpho-neuraminic acid | Neu5Ac8S | 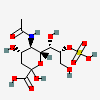 | 129681273 | 480493155 | 5, 19, 36, 37 | ||
| 5-N-Acetyl-4-O-acetyl-8-O-sulpho-neuraminic acid | Neu4,5Ac28S | 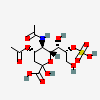 | 140507948 | 480493156 | 5 | ||
| 5-N-Acetyl-9-O-phospho-neuraminic acidd | Neu5Ac9P | 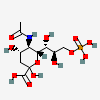 | 440962 | 480493157 | 38 | ||
| 5-N-Acetyl-2-deoxy-2,3-didehydro-neuraminic acidd,i | Neu2en5Ac | 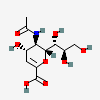 | 65309 | 480493158 | 8, 17, 20, 30, 39, 40, 41 | ||
| 5-N-Acetyl-9-O-acetyl-2-deoxy-2,3-didehydro-neuraminic acidd,i | Neu2en5,9Ac2 | 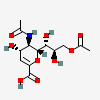 | 102169087 | 480493159 | 42, 43 | ||
| 5-N-Acetyl-2-deoxy-2,3-didehydro-9-O-lactyl-neuraminic acidd,i | Neu2en5Ac9Lt |  | 168009046 | 480493160 | 42, 43 | ||
| 5-N-Acetyl-2,7-anhydro-neuraminic acidd,i | Neu2,7an5Ac |  | 449401 | 480493161 | 8, 20, 42, 44, 45, 46 | ||
| 5-N-Acetyl-4,8-anhydro-neuraminic acide,i | Neu4,8an5Ac | 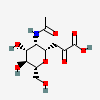 | 14283565 | 480493162 | 18, 19, 47, 48 | ||
| 5-N-Acetyl-neuraminic acid 1,7-lactonei | Neu1,7lactone5Ac |  | 102027926 | 480493163 | 5, 49, 50 | ||
| 5-N-Acetyl-9-O-acetyl-neuraminic acid 1,7-lactonei | Neu1,7lactone5,9Ac2 | 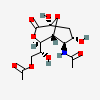 | 168009047 | 480493164 | 5 | ||
| 5-N-Acetyl-4,9-di-O-acetyl-neuraminic acid 1,7-lactonei | Neu1,7lactone4,5,9Ac3 | 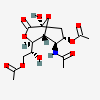 | 168009048 | 480493165 | 5 | ||
| 1-Tauryl 5-N-acetyl-neuraminic amide | Neu5Ac1Tau | 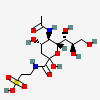 | 168009049 | 480493166 | 51 | ||
| 5-N-Glycolyl-neuraminic acid (N-Glycolylneuraminic acid) Neu5Gc | Neu5Gc | 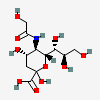 | 440001 | 252089091 | 5, 8, 10, 13, 14, 16, 17, 18, 19, 20, 52, 53, 54 | [CSDB] | |
| 4-O-Acetyl-5-N-glycolyl-neuraminic acid | Neu4Ac5Gc |  | 129674796 | 480493167 | 8, 14, 17, 18, 19, 20, 28 | [CSDB] | |
| 7-O-Acetyl-5-N-glycolyl-neuraminic acid | Neu7Ac5Gc | 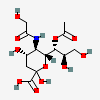 | 140387950 | 480493168 | 8, 17, 19, 20, 24 | ||
| 8-O-Acetyl-5-N-glycolyl-neuraminic acid | Neu8Ac5Gc | 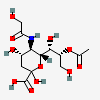 | 140507946 | 480493169 | 19, 42 | ||
| 9-O-Acetyl-5-N-glycolyl-neuraminic acid | Neu9Ac5Gc | 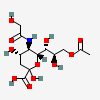 | 129775298 | 480493170 | 5, 8, 14, 16, 17, 18, 19, 20, 24 | ||
| 4,7-Di-O-acetyl-5-N-glycolyl-neuraminic acid | Neu4,7Ac25Gc | 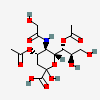 | 140507953 | 480493171 | 5 | ||
| 4,9-Di-O-acetyl-5-N-glycolyl-neuraminic acid | Neu4,9Ac25Gc |  | 129800727 | 480493172 | 5 | ||
| 7,9-Di-O-acetyl-5-N-glycolyl-neuraminic acid | Neu7,9Ac25Gc | 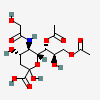 | 140387948 | 480493173 | 5, 8, 17, 18, 19, 20, 24 | ||
| 8,9-Di-O-acetyl-5-N-glycolyl-neuraminic acid | Neu8,9Ac25Gc | 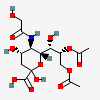 | 129716700 | 480493174 | 5, 8, 17, 18, 19, 20, 24 | ||
| 4,7,9-Tri-O-acetyl-5-N-glycolyl-neuraminic acid | Neu4,7,9Ac35Gc | 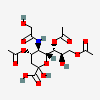 | 168009050 | 480493175 | 5 | ||
| 7,8,9-Tri-O-acetyl-5-N-glycolyl-neuraminic acid | Neu7,8,9Ac35Gc | 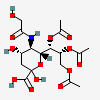 | 129716704 | 480493176 | 8, 17, 18, 20, 24 | ||
| 4,7,8,9-Tetra-O-acetyl-5-N-glycolyl-neuraminic acid | Neu4,7,8,9Ac45Gc | 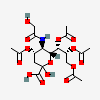 | 168009051 | 480493177 | 5 | ||
| 5-N-Glycolyl-9-O-lactyl-neuraminic acid | Neu5Gc9Lt |  | 140372591 | 480493178 | 5, 19, 42 | ||
| 4-O-Acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu4Ac5Gc9Lt | 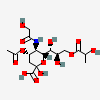 | 140387947 | 480493179 | 5 | ||
| 7-O-Acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu7Ac5Gc9Lt | 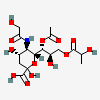 | 168009052 | 480493180 | 5 | ||
| 8-O-Acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu8Ac5Gc9Lt |  | 140507950 | 480493181 | 5 | ||
| 4,7-Di-O-acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu4,7Ac25Gc9Lt | 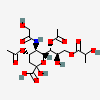 | 140507945 | 480493182 | 5 | ||
| 7,8-Di-O-acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu7,8Ac25Gc9Lt | 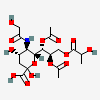 | 140507955 | 480493183 | 5 | ||
| 5-N-Glycolyl-8-O-methyl-neuraminic acid | Neu5Gc8Me | 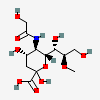 | 129800653 | 480493184 | 8, 18, 19, 20, 33, 55, 56 | ||
| 4-O-Acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu4Ac5Gc8Me | 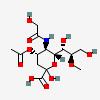 | 168009053 | 480493185 | 57 | ||
| 7-O-Acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu7Ac5Gc8Me |  | 168009054 | 480493186 | 19, 57 | ||
| 9-O-Acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu9Ac5Gc8Me | 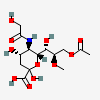 | 129829096 | 480493187 | 8, 18, 19, 20, 33, 42 | ||
| 4,7-Di-O-acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu4,7Ac25Gc8Me | 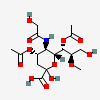 | 168009055 | 480493188 | 57 | ||
| 7,9-Di-O-acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu7,9Ac25Gc8Me | 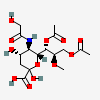 | 129829113 | 480493189 | 18 | ||
| 5-N-Glycolyl-9-O-methyl-neuraminic acid | Neu5Gc9Me | 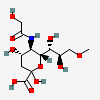 | 168009056 | 480493190 | 34, 58 | ||
| 5-N-Glycolyl-8-O-sulpho-neuraminic acid | Neu5Gc8S |  | 129681276 | 480493191 | 5, 19, 36, 59 | ||
| 5-N-Glycolyl-9-O-sulpho-neuraminic acid | Neu5Gc9S |  | 140507947 | 480493192 | 60 | ||
| 5-N-(O-Acetyl)glycolyl-neuraminic acid | Neu5(Gc2Ac) | 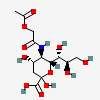 | 129752159 | 480493193 | 8, 20, 61 | ||
| 5-N-(O-Methyl)glycolyl-neuraminic acid | Neu5(Gc2Me) | 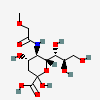 | 168009057 | 480493194 | 62 | ||
| 2-Deoxy-2,3-didehydro-5-N-glycolyl-neuraminic acidd,i | Neu2en5Gc | 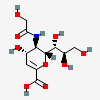 | 3082514 | 480493195 | 8, 20, 43, 63 | ||
| 9-O-Acetyl-2-deoxy-2,3-didehydro-5-N-glycolyl-neuraminic acidd,i | Neu2en9Ac5Gc | 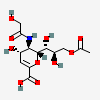 | 162884378 | 480493196 | 43 | ||
| 2-Deoxy-2,3-didehydro-5-N-glycolyl-9-O-lactyl-neuraminic acidd,i | Neu2en5Gc9Lt |  | 168009058 | 480493197 | 42, 43 | ||
| 2-Deoxy-2,3-didehydro-5-N-glycolyl-8-O-methyl-neuraminic acidd,i | Neu2en5Gc8Me | 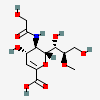 | 168009059 | 480493198 | 42 | ||
| 2,7-Anhydro-5-N-glycolyl-neuraminic acidd,i | Neu2,7an5Gc | 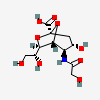 | 101638299 | 480493199 | 20, 42, 45 | ||
| 2,7-Anhydro-5-N-glycolyl-8-O-methyl-neuraminic acidd,i | Neu2,7an5Gc8Me |  | 168009060 | 480493200 | 42 | ||
| 4,8-Anhydro-5-N-glycolyl-neuraminic acide,i | Neu4,8an5Gc | 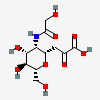 | 168009061 | 480493201 | 19 | ||
| 5-N-Glycolyl-neuraminic acid 1,7-lactonei | Neu1,7lactone5Gc | 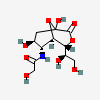 | 168009062 | 480493202 | 5 | ||
| 9-O-Acetyl-5-N-glycolyl-neuraminic acid 1,7-lactonei | Neu1,7lactone9Ac5Gc |  | 168009063 | 480493203 | 5 | ||
| 7-Acetamido-9-O-acetyl-7-deoxy-5-N-glycolyl-neuraminic acidf,g | Neu9Ac5Gc7NAc | 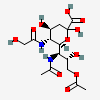 | 168009064 | 480493204 | 5 | ||
| 7-Acetamido-8,9-di-O-acetyl-7-deoxy-5-N-glycolyl-neuraminic acidf,g,h | Neu8,9Ac25Gc7NAc |  | 168009065 | 480493205 | 5 | ||
| 3-Deoxy-D-glycero-D-galacto-non-2-ulosonic acid / 2-keto-3-deoxy-nononic acid (Kdn) | Kdn | 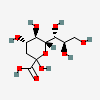 | 13991616 | 252089089 | 5, 19, 64, 65, 66 | [CSDB] | |
| 5-O-Acetyl-2-keto-3-deoxy-nononic acid | Kdn5Ac | 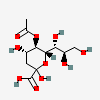 | 168009066 | 480493206 | 5, 19 | ||
| 7-O-Acetyl-2-keto-3-deoxy-nononic acid | Kdn7Ac | 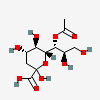 | 168009067 | 480493207 | 5, 19 | ||
| 8-O-Acetyl-2-keto-3-deoxy-nononic acid | Kdn8Ac | 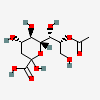 | 168009068 | 480493208 | 67 | ||
| 9-O-Acetyl-2-keto-3-deoxy-nononic acid | Kdn9Ac |  | 14633447 | 480493209 | 5, 19, 68 | ||
| 4,5-Di-O-acetyl-2-keto-3-deoxy-nononic acid | Kdn4,5Ac2 | 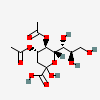 | 168009069 | 480493210 | 5 | ||
| 4,7-Di-O-acetyl-2-keto-3-deoxy-nononic acid | Kdn4,7Ac2 | 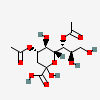 | 168009070 | 480493211 | 5 | ||
| 5,9-Di-O-acetyl-2-keto-3-deoxy-nononic acid | Kdn5,9Ac2 | 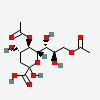 | 168009071 | 480493212 | 19 | ||
| 7,9-Di-O-acetyl-2-keto-3-deoxy-nononic acid | Kdn7,9Ac2 | 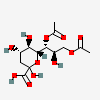 | 168009072 | 480493213 | 5, 19 | ||
| 8,9-Di-O-acetyl-2-keto-3-deoxy-nononic acid | Kdn8,9Ac2 | 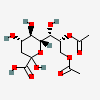 | 168009073 | 480493214 | 5 | ||
| 2-Keto-3-deoxy-4-O-methyl-nononic acid | Kdn4Me | 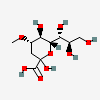 | 168009074 | 480493215 | 69 | [CSDB] | |
| 2-Keto-3-deoxy-5-O-methyl-nononic acid | Kdn5Me | 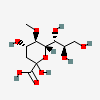 | 168009075 | 480493216 | 70 | ||
| 2-Keto-3-deoxy-9-O-methyl-nononic acid | Kdn9Me |  | 168009076 | 480493217 | 71 | [CSDB] | |
| (R)-7,9-O-[1-Carboxyethylidene]-2-keto-3-deoxy-nononic acid | Kdn7,9PyrR | 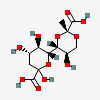 | 168009077 | 480493218 | 72 | [CSDB] | |
| 2-Keto-3-deoxy-9-O-phospho-nononic acidd | Kdn9P |  | 124037361 | 480493219 | 73 |
a Present only in bound form, and considered to be derived from bound Neu5Ac in an enzymatic de-N-acetylation/re-N-acetylation cycle.
b Present only in bound form, and considered to be derived from bound Neu in an enzymatic dehydration reaction, catalyzed by a so-called sialic acid cyclase. Neu1,5lactam was initially called ‘cyclic sialic acid’.
c Lactyl = L-Lactyl.
d Present only in free form.
e Neu4,8an5Ac does not occur as such in nature. It is assumed to be formed under hydrolytic conditions from bound Neu4,5Ac2.
f The D-glycero-D-galacto configuration has not been verified by the authors.
g Compared with Ref. 5, 7Am has been changed into 7NAc.
h Neu4,9Ac27Am5Gc in the text of Ref. 5 is probably a typing error for Neu8,9Ac27Am5Gc.
i 'en' (didehydro) is 2-3, 'an' (anhydro) is 2-7, 'on' (lactone) is 1-7, and 'am' (lactam) is 1-5, by default, unless specify otherwise in legend, e.g. 4-8.
Table 2 Reported structures of naturally occurring 5,7-Diamino-3,5,7,9-tetradeoxy-non-2-ulosonic acid derivatives, and some related structures based on NMR and/or mass spectrometry reports.
(updated survey since the publication of R. Schauer & J.P. Kamerling, Exploration of the Sialic Acid World, Adv. Carbohydr. Chem. Biochem. 2018, 75, 1-213; combined with SNFG symbols) (Download TSV data file)
| Name | Abbreviation | Structure | CID | SID | SNFG | References | Database Links |
|---|---|---|---|---|---|---|---|
| 5,7-Diamino-3,5,7,9-tetradeoxy-L-glycero-L-manno-non-2-ulosonic acid / pseudaminic acid (Pse) | Pse | 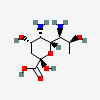 | 101137651 | 252089088 | |||
| 5,7-Di-N-acetyl-pseudaminic acid | Pse5,7Ac2 |  | 56927740 | 480493220 | 74, 75 | [CSDB] | |
| 5,7-Di-N-acetyl-4-O-acetyl-pseudaminic acid | Pse4,5,7Ac3 | 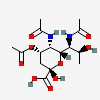 | 168009078 | 480493221 | 76 | [CSDB] | |
| 5,7-Di-N-acetyl-8-O-acetyl-pseudaminic acid | Pse5,7,8Ac3 | 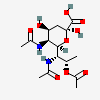 | 168009079 | 480493222 | 75 | ||
| 5,7-Di-N-acetyl-8-O-glycyl-pseudaminic acid | Pse5,7Ac28Gly | 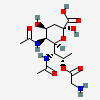 | 168009080 | 480493223 | 77 | ||
| 5,7-Di-N-glyceryl-pseudaminic acid | Pse5,7Gr2 | 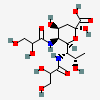 | 168009081 | 480493224 | 75 | ||
| 5-N-Acetimidoyl-7-N-acetyl-pseudaminic acid | Pse7Ac5Am |  | 168009082 | 480493225 | 75, 78 | [CSDB] | |
| 5-N-Acetimidoyl-7-N-acetyl-8-O-acetyl-pseudaminic acid | Pse7,8Ac25Am | 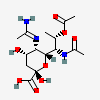 | 168009083 | 480493226 | 79 | ||
| 5-N-Acetimidoyl-7-N-acetyl-8-O-(N-acetyl-glutaminyl)-pseudaminic acid | Pse7Ac5Am8(Gln2Ac) | 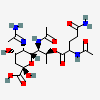 | 168009084 | 480493227 | 77 | ||
| 5-N-Acetyl-7-N-formyl-pseudaminic acid | Pse5Ac7Fo | 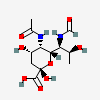 | 163114430 | 480493228 | 80, 81 | [CSDB] | |
| 5-N-Acetyl-7-N-L-glyceryl-pseudaminic acid | Pse5Ac7Gr | 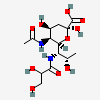 | 168009085 | 480493229 | 82 | ||
| 5-N-Acetyl-7-N-[(R)-3-hydroxybutyryl]-pseudaminic acid | Pse5Ac7(3RHb) | 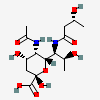 | 168009086 | 480493230 | 80, 83, 84 | [CSDB] | |
| 5-N-Acetyl-7-N-[(R)-3-hydroxybutyryl]-4-O-acetyl-pseudaminic acid | Pse4,5Ac27(3RHb) | 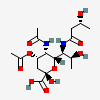 | 168009087 | 480493231 | 85, 86 | [CSDB] | |
| 5-N-Acetyl-7-N-[(S)-3-hydroxybutyryl]-pseudaminic acid | Pse5Ac7(3SHb) |  | 168009088 | 480493232 | 84 | [CSDB] | |
| 5-N-Acetyl-7-N-(4-hydroxybutyryl)-pseudaminic acid | Pse5Ac7(4Hb) | 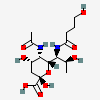 | 168009089 | 480493233 | 87 | [CSDB] | |
| 5-N-Acetyl-7-N-(3,4-dihydroxybutyryl)-pseudaminic acid | Pse5Ac7(3,4Hb) | 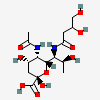 | 168009090 | 480493234 | 88 | [CSDB] | |
| 7-N-Acetimidoyl-5-N-acetyl-pseudaminic acid | Pse5Ac7Am | 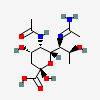 | 168009091 | 480493235 | 89 | ||
| 5-N-Acetimidoyl-7-N-glyceryl-pseudaminic acida | Pse5Am7Gr | 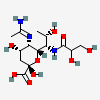 | 168009092 | 480493236 | 90 | [CSDB] | |
| 7-N-Acetimidoyl-5-N-(2,3-di-O-methyl-glyceryl)-pseudaminic acid | Pse7Am5(Gr2,3Me2) | 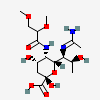 | 168009093 | 480493237 | 91 | ||
| 7-N-Acetyl-5-N-(3-hydroxybutyryl)-pseudaminic acid | Pse7Ac5(3Hb) |  | 168009094 | 480493238 | 92 | [CSDB] | |
| 7-N-Acetyl-5-N-(2,3-di-O-methyl-glyceryl)-pseudaminic acid | Pse7Ac5(Gr2,3Me2) |  | 168009095 | 480493239 | 91 | ||
| 7-N-Formyl-5-N-[(R)-3-hydroxybutyryl]-pseudaminic acid | Pse7Fo5(3RHb) |  | 168009096 | 480493240 | 80, 93, 94 | [CSDB] | |
| 5,7-Diamino-3,5,7,9-tetradeoxy-D-glycero-D-galacto-non-2-ulosonic acid / legionaminic acid (Leg) | Leg | 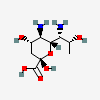 | 70678967 | 252089090 | [CSDB] | ||
| 5,7-Di-N-acetyl-legionaminic acidb | Leg5,7Ac2 | 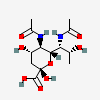 | 21572994 | 480493241 | 95, 96, 97, 98 | [CSDB] | |
| 5,7-Di-N-acetyl-4-O-acetyl-legionaminic acid | Leg4,5,7Ac3 | 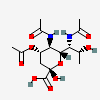 | 168009097 | 480493242 | 76 | [CSDB] | |
| 5,7-Di-N-acetyl-8-amino-8-deoxy-legionaminic acid | Leg5,7Ac28N | 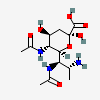 | 168009098 | 480493243 | 99 | [CSDB] | |
| 5-N-Acetimidoyl-7-N-acetyl-legionaminic acidb | Leg7Ac5Am |  | 102086088 | 480493244 | 95, 97, 98 | [CSDB] | |
| 5-N-Acetimidoyl-7-N-acetyl-8-O-acetyl-legionaminic acidc | Leg7,8Ac25Am | 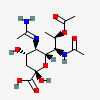 | 168009099 | 480493245 | 95, 100 | [CSDB] | |
| 5-N-Acetimidoyl-7-N-acetyl-5-N-methyl-legionaminic acid | Leg7Ac5Am5Me | 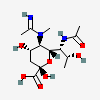 | 168009100 | 480493246 | 101 | [CSDB] | |
| 5-N-(N-Methyl-acetimidoyl)-7-N-acetyl-legionaminic acid | Leg7Ac5AmMe |  | 168009101 | 480493247 | 97, 101 | [CSDB] | |
| 5-N-(N,N-Dimethyl-acetimidoyl)-7-N-acetyl-legionaminic acid | Leg7Ac5AmMe2 |  | 168009102 | 480493248 | 101 | ||
| 5-N-Acetimidoyl-7-N-acetyl-8-O-acetyl-5-N-methyl-legionaminic acid | Leg7,8Ac25Am5Me | 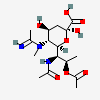 | 168009103 | 480493249 | 100 | ||
| 5-N-(N,N-Dimethyl-acetimidoyl)-7-N-acetyl-8-O-acetyl-legionaminic acid | Leg7,8Ac25AmMe2 |  | 168009104 | 480493250 | 100 | ||
| 5-N-Acetyl-7-N-(N-acetyl-D-alanyl)-legionaminic acid | Leg5Ac7(Ala2Ac) | 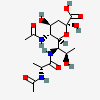 | 10993159 | 480493251 | 96 | ||
| 5-N-Acetyl-7-N-(D-alanyl)-legionaminic acid | Leg5Ac7Ala |  | 154573042 | 480493252 | 102 | [CSDB] | |
| 7-N-Acetyl-5-N-formyl-legionaminic acid | Leg7Ac5Fo | 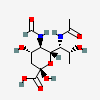 | 168009105 | 480493253 | 103 | [CSDB] | |
| 7-N-Acetyl-5-N-[(S)-3-hydroxybutyryl]-legionaminic acidd | Leg7Ac5(3SHb) | 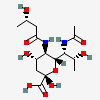 | 168009106 | 480493254 | 95, 98 | [CSDB] | |
| 7-N-Acetyl-5-N-(N-methyl-5-glutamyl)-legionaminic acid | Leg7Ac5(5Glu2Me) | 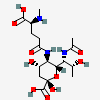 | 168009107 | 480493255 | 104 | ||
| 5,7-Diamino-3,5,7,9-tetradeoxy-D-glycero-D-talo-non-2-ulosonic acid / 4-epi-legionaminic acid (4eLeg) | 4eLeg | 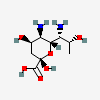 | 126961780 | 252089092 | |||
| 5,7-Di-N-acetyl-4-epi-legionaminic acid | 4eLeg5,7Ac2 |  | 168009108 | 480493256 | 105, 98 | ||
| 5,7-Di-N-acetyl-8-O-acetyl-4-epi-legionaminic acide | 4eLeg5,7,8Ac3 | 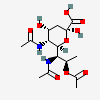 | 168009109 | 480493257 | 95 | ||
| 5-N-Acetimidoyl-7-N-acetyl-4-epi-legionaminic acid | 4eLeg7Ac5Am |  | 168009110 | 480493258 | 106, 98 | ||
| 5-N-Acetimidoyl-7-N-acetyl-8-O-acetyl-4-epi-legionaminic acid | 4eLeg7,8Ac25Am | 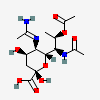 | 168009111 | 480493259 | 106 | ||
| 5,7-Diamino-3,5,7,9-tetradeoxy-L-glycero-D-galacto-non-2-ulosonic acid / 8-epi-legionaminic acid (8eLeg) | 8eLeg |  | 168009112 | 252090150 | |||
| 5,7-Di-N-acetyl-8-epi-legionaminic acidf | 8eLeg5,7Ac2 |  | 154573043 | 480493260 | 107, 108, 109, 98 | [CSDB] | |
| 5,7-Di-N-acetyl-8-O-acetyl-8-epi-legionaminic acid | 8eLeg5,7,8Ac3 | 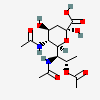 | 168009113 | 480493261 | 110 | [CSDB] | |
| 5-N-Acetimidoyl-7-N-acetyl-8-epi-legionaminic acid | 8eLeg7Ac5Am | 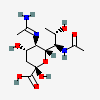 | 168009114 | 480493262 | 111, 98 | [CSDB] | |
| 7-N-Acetimidoyl-5-N-acetyl-8-epi-legionaminic acid | 8eLeg5Ac7Am |  | 168009115 | 480493263 | 67, 98 | ||
| 7-N-Acetimidoyl-5-N-acetyl-8-O-acetyl-8-epi-legionaminic acid | 8eLeg5,8Ac27Am | 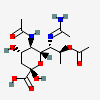 | 168009116 | 480493264 | 67 | [CSDB] | |
| 7-N-Acetyl-5-N-[(R)-3-hydroxybutyryl]-8-epi-legionaminic acidf | 8eLeg7Ac5(3RHb) | 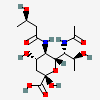 | 168009117 | 480493265 | 95, 98 | [CSDB] | |
| 7-N-Acetyl-5-N-(4-hydroxybutyryl)-8-epi-legionaminic acide | 8eLeg7Ac5(4Hb) | 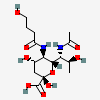 | 168009118 | 480493266 | 95, 98 | [CSDB] | |
| 5,7-Diamino-3,5,7,9-tetradeoxy-L-glycero-L-altro-non-2-ulosonic acid / acinetaminic acid (Aci) | Aci | 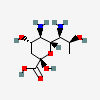 | 126961779 | 252089094 | |||
| 5,7-Di-N-acetyl-acinetaminic acid | Aci5,7Ac2 | 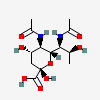 | 102515389 | 480493267 | 112 | [CSDB] | |
| 5,7-Diamino-3,5,7,9-tetradeoxy-D-glycero-L-altro-non-2-ulosonic acid / 8-epi-acinetaminic acid (8eAci) | 8eAci | 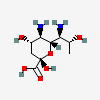 | 168009119 | 480493268 | |||
| 5,7-Di-N-acetyl-8-epi-acinetaminic acid | 8eAci5,7Ac2 | 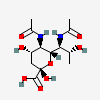 | 168009120 | 480493269 | 113 | [CSDB] | |
| Some related 9-deoxy-non-2-ulosonic acids | |||||||
| 5- or 7-Acetamido-,7- or 5-(3-hydroxybutyramido)-5,7,9-trideoxy-non-2-ulosonic acid | 114 | ||||||
| 5-Acetamido-7-[(S)-3-hydroxybutyramido]-8-amino-3,5,7,8,9-pentadeoxy-L-glycero-L-manno- or D-glycero-L-manno-non-2-ulosonic acid | 115 | ||||||
| 5-Acetamidino-3,5,9-trideoxy-L-glycero-L-gluco-non-2-ulosonic acid (tentatively assigned chirality; trivial name: fusaminic acid) | 116 | [CSDB] | |||||
| 5-Acetamidino-4-O-acetyl-3,5,9-trideoxy-L-glycero-L-gluco-non-2-ulosonic acid (tentatively assigned chirality) | 116 | [CSDB] | |||||
| 5-Acetamidino-7-acetamido-3,5,7,9-tetradeoxy-D-glycero-L-gluco-non-2-ulosonic acid (tentatively assigned chirality) | 117 | [CSDB] |
a Pse5Am7Gr was earlier reported as Pse5Am7Gc118
b Initially assigned with the D-glycero-L-galacto and later with the L-glycero-D-galacto configuration.98, 107, 108, 119
c Initially assigned with the D-glycero-L-galacto 120, 121, 122 and later with the L-glycero-D-galacto107, 108,98 configuration.
d Initially assigned with the L-glycero-D-galacto configuration.119, 98
e Initially assigned with the L-glycero-D-talo configuration.123, 98
f Initially assigned with the D-glycero-L-galacto configuration.98, 124, 125, 126