NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cappellini MD, Farmakis D, Porter J, et al., editors. 2021 Guidelines: For the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 4th edition. Nicosia (Cyprus): Thalassaemia International Federation; 2023.

2021 Guidelines: For the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 4th edition.
Show detailsIntroduction
Iron overload and viral hepatitis are the two main causes of liver disease in patients with thalassaemia leading to chronic inflammation, fibrosis and ultimately cirrhosis.
The approach to liver disease includes clinical parameters, biochemical/serological/molecular tests and imaging techniques in order to identify the aetiological factor(s) and assess the severity of the injury.
Clinical examination may reveal signs of systemic iron excess such as skin pigmentation and hepatomegaly while in later stages stigmata of chronic liver disease (i.e. palmar erythema, spider naevi), ascites and encephalopathy may follow.
Laboratory tests may show moderate elevations (2-3 times higher than the upper limit of normal) of the aminotransferases, aspartate transaminase (AST) and alanine transaminase (ALT) and a mild increase of alkaline phosphatase and gamma glutamyl transferase (γGT). In the presence of severe hepatic impairment, prolonged prothrombin time, low albumin and high serum bilirubin are found.
With regard to imaging techniques, ultrasonography (US) remains the gold standard to evaluate hepatic morphology and to recognise signs of fibrosis and cirrhosis. Portal hypertension can be also assessed by duplex ultrasonography or spectral Doppler imaging and colour Doppler imaging (Procopet & Berzigotti, 2017). Hepatic transient elastography (TE), a pulse-echo ultrasound technique, has replaced biopsy as a non-invasive diagnostic method for staging liver fibrosis. Liver stiffness, measured by TE, has also been shown to correlate with stages of fibrosis in patients with thalassaemia (Ferraioli et al., 2016; Paparo et al., 2013; Di Marco et al., 2010a), although specific cut-off values for the stage of fibrosis need to be defined. Moreover, it is believed that iron overload may affect the accuracy of TE in assesing liver fibrosis (Fraquelli et al., 2010). As compared to liver biopsy which is the gold standard examination for the evaluation of liver fibrosis but consists an invasive techniq with potential complications, TE is an easy-to-perform diagnostic tool with moderate to high accuracy, especially in thalassaemic patients with chronic HCV infection (Poustchi et al., 2013).
A) Hepatic Iron Overload
1. Pathophysiology
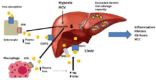
Hepatocytes are the main site of iron storage and also the principal site of synthesis of hepcidin, a hormone responsible for the regulation of iron transport to the extracellular space through activation of the cellular iron exporter, ferroportin. In cases of thalassaemia, the excess iron due to frequent transfusions initially accumulates in macrophages and later in hepatocytes, while hypoxia caused by dyserythropoiesis intensifies erythropoietin production leading to further suppression of hepcidin production and increased transferrin bound iron (TBI) and non-transferrin bound iron (NTBI) plasma levels. (Chaston et al., 2011).
Excessive levels of iron in the blood stream exceed the capacity of plasma transferrin to bind iron and lead to the appearance of NTBI in the plasma which consists a highly toxic iron species. The liver rapidly clears NTBI from the serum through divalent metal transporter 1 (DMT1) and incorporates it into ferritin. However, once the protective storage efficiency of ferritin is exceeded, unbound iron accumulates in the hepatocytes and leads to severe oxidative stress and overproduction of toxic reactive oxygen species (ROS), which cause lipid peroxidation and protein damage (Figure 1). The subsequent hepatic inflammation and necrosis lead to fibrosis and cirrhosis (Sikorska, Bernat & Wroblewska, 2016).Development of fibrosis in the absence of inflammation has also been found to be associated with iron accumulation in the liver and relates to direct activation of stellate cells (Philippe, Ruddell & Ramm, 2007).
2. Iron overload monitoring
Magnetic resonance imaging (MRI) R2 or R2* using liver iron content (LIC) (mg of iron/g dry weight) is considered the method of choice for iron load monitoring in thalassaemic patients. A yearly LIC assessment should be performed in all patients in order to monitor chelation therapy effectiveness (Brittenham, 2011). LIC values greater than 7mg Fe/g dry weight and 15mg/g dry weight are associated with moderate and severe iron overload respectively (Allali et al., 2017) while LIC>16mg/g dry weight is associated with an increased risk of hepatic fibrosis. MRI iron assessment of the liver is also mandatory as a pre- and post-transplant evaluation in cases of haematopoietic stem cell transplantation (HSCT) (Mavrogeni et al., 2018).
Serum ferritin (SF) is the most widely used marker of total body iron load. SF values higher than 2000 ng/ml are associated with liver iron overload (Krittayaphong et al., 2018). However, SF measurement has low specificity and should be interpreted with caution since it can be elevated also in the case of concomitant inflammation, malignancy or oxidative stress (Chirico et al., 2015; Puliyel et al., 2014).
Transient elastography has been used for the assessment of liver fibrosis in thalassaemic patients with initially promising results. Its value as a means of estimation of iron overload has been examined in recent studies. TE values were found to be strongly correlated to MRI T2* results (Pipaliya et al., 2017). LIC values, SF levels and TE stiffness measurements in patients with sickle cell anaemia were also correlated and found to improve after successful chelation therapy (Delicou et al., 2018), although this correlation was not confirmed by other studies (Ou et al., 2017). Further studies focusing on the effectiveness of TE in assessing liver iron overload need to be conducted in order to draw robust conclusions.
3. Treatment
Three chelators are currently available for the treatment of iron overload in thalassaemic patients: deferoxamine (DFO), deferiprone (DFP) and deferasirox (DFX). They are all proven to effectively reduce LIC (Maggio et al., 2011, 2002; Taher et al., 2009), while specifically DFX may have a positive effect on liver fibrosis as well (Deugnier et al., 2011; Maira et al., 2017; Sousos et al., 2018).
B) Viral Hepatitis
Hepatitis C virus infection
Chronic infection with hepatitis C virus (HCV) in combination with iron load hepatotoxicity remains a major risk factor for acceleration of liver fibrosis in thalassaemic patients. Antiviral treatment used to be quite challenging, mainly due to the haemolytic effect of ribavirin and interferon (IFN)-induced cytopenias (Kowdley, 2005). Recent advances in HCV therapies have improved treatment success rates and minimised adverse events significantly.
1. Hepatitis C virus epidemiology
The prevalence of HCV infection among thalassaemic patients shows great variation in published data (Di Marco et al., 2010b), ranging between 19 and 75% and depending on the region where it was studied(Behzadifar, Gorji & Bragazzi, 2018; Jang et al., 2017; Kountouras et al., 2013; Triantos et al., 2013).The most frequent HCV genotype (GT) in thalassaemic patients is GT 1b, reflecting the distribution of genotypes in the general population per geographical area (Di Marco et al., 2010b; Dodd, Notari & Stramer, 2002). More recent epidemiological studies in specific eastern Mediterranean countries describe the increase of GT3 prevalence (Ahmadi-Ghezeldasht et al., 2018) as well. The implementation of systematic blood donor screening with molecular methods in the last two decades has dramatically decreased HCV transmission through transfusions in Western countries (Dodd et al.2002), although such methods may not yet be extensively applied in developing countries.
2. Hepatitis C virus infection, diagnosis and treatment
All thalassaemic patients who were transfused before 1991 should be screened for HCV infection. HCV diagnosis requires two steps: a) blood testing for anti-HCV antibodies, b) HCV-RNA by polymerase chain reaction (PCR) testing in the case of positive anti-HCV result (Figure 2). The diagnosis of chronic HCV infection must be followed by fibrosis stage evaluation with TE, hepatic function assessment and genotype determination when a pangenotypic regimen is not available in order to select the best treatment option (EASL HCV Guidelines, 2020).

Figure 2
Hepatitis C virus (HCV) diagnosis algorithm.
The HCV treatment landscape has changed dramatically in recent years. Direct acting anti-viral agents (DAAs) have emerged (Table 1), yielding > 95% rates of sustained virological response (SVR) which is the equivalent of complete cure. DAAs have replaced interferon in the treatment of HCV infection and have combined efficacy with a very safe profile. Treatment duration varies between 8 and12 weeks, with ribavirin co-administration not being needed in many cases. Pangenotypic regimens that provide high SVR rates across genotypes are also available and have further simplified therapy (Table 1). As a result, the indication for HCV treatment in the thalassaemic population is similar to that of the general population (EASL HCV Guidelines, 2020). Specific phase 3 (Hézode et al., 2017), randomised (Mangia et al., 2017) and real-life studies (Sinakos et al., 2017) have confirmed DAAs effectiveness in thalassaemic patients, showing SVR rates comparable to those in the general population and more significantly without any additional safety concerns. It should be noted that before treatment initiation, drug-drug interactions between concomitant medications and DAAs should be checked. Liverpool University site for Hepatitis Drug interactions (https://hep-druginteractions.org/query) is a very useful tool for everyday clinical practice. In general, co-administration of anti-viral agents with chelation treatment is not contra-indicated, while specific caution should be taken with certain anti-arrhythmic drugs that share common metabolic paths with DAAs with the consequence that co-administration may affect drug levels (EASL HCV Guidelines, 2020).
Table 1
Summary of approved Direct Anti-viral Agents(DAAs) per Hepatitis C Virus Genotype (EASL 2020).
Hepatitis B virus infection
Chronic hepatitis B virus (HBV) infection was reported to be present in 5% of thalassaemic patients based on an Italian registry (Borgna-Pignatti et al., 2014). This rate could be higher in countries where blood donors are not adequately assessed. Diagnosis is made by the presence of detectable HBV surface antigen (HBsAg) in serum for more than 6 months. HBV DNA levels in serum detected by PCR, presence of HBV e antigen (HBeAg), fibrosis stage and transaminase levels guide the decision for treatment. Currently approved drugs for treatment of chronic HBV infection include pegylated interferons and oral nucleoside/nucleotide analogues (EASL HBV Guidelines, 2017), but interferons should be avoided due to their myelosuppressive effect (Kowdley, 2005). Prevention is mandatory and HBV vaccination is strongly recommended. Immunisation of thalassaemic children for HBV has been proven to be effective without any tolerance issues (Sharifi, Milani & Shooshtari, 2010).
Hepatitis E virus (HEV) infection
Hepatitis E virus (HEV) infection rates have been increasing lately, even in high-income countries (Capai, Charrel & Falchi, 2018). Despite the fact that the predominant route of HEV transmission is consumption of contaminated food, it is estimated that 1/5000 blood donations could be contaminated (Capai, Charrel & Falchi, 2018). Since acute HEV infection may have severe complications in patients with pre-existing liver disease, testing for IgM anti-HEV antibodies and HEV viral load assessment should be performed in cases of acute hepatitis or unexplained flares of chronic liver disease (EASL HEV Guidelines, 2018) in thalassaemic patients. A case of acute hepatitis E infection in a thalassaemic patient has been already reported (Politis et al., 2018). In severe cases, treatment guidelines include ribavirin and IFN, although further investigation is needed (EASL HEV Guidelines, 2018). Universal screening of blood donors is not a worldwide strategy due to cost and differences in the frequency of immunoglobulin (Ig) G anti-HEV antibodies in the general population.
C) Hepatocellular Carcinoma
1. Incidence and Pathophysiology
The incidence of hepatocellular carcinoma (HCC) in thalassaemic patients has increased lately due to the prolonged survival achieved by effective iron chelation therapies and decrease of heart-associated complications (Borgna-Pignatti et al., 2014). Co-existence of specific risk factors such as iron overload and HCV or HBV infection contribute to the development of HCC and provide an explanation for the reported younger age of HCC diagnosis (Mancuso et al., 2006) in this population. Furthermore, there are reported cases of HCC in non-cirrhotic thalassaemic patients (Deugnier & Turlin, 2001; Papadopoulos et al., 2020).HCV infection and iron overload are reported to work in a synergistic way towards HCC development. More specifically, it has been described that HCV induces reduction of serum hepcidin (Girelli et al., 2009) promoting further accumulation of total iron, while unbound iron has been connected to carcinogenesis through the production of ROS. Moreover, frequent blood transfusions cause continuous antigenic stimulation which could potentially have an immunomodulatory effect (Refaai & Blumberg, 2013).
2. Screening for HCC
Screening patients with thalassaemia for HCC has a pivotal role in their clinical management. Abdominal US should be performed every 6 months in cirrhotic patients (EASL HCC Guidelines, 2018), while alpha fetoprotein (AFP) is not considered a reliable marker in general and especially in thalassaemic patients (Fragatou, Tsourveloudis & Manesis, 2010). The early onset of HCC and the observation of HCC cases even in non-cirrhotic patients indicate that biannual screening should be extended not only to cirrhotic patients, but also to every patient with high risk factors such as HCV and/or HBV infection, non-transfusion-dependent thalassaemia (NTDT) with LIC≥ 5 mg Fe/g dry weight, transfusion-dependent thalassaemia (TDT) with LIC≥ 7 mg Fe/g dry weight or serum ferritin ≥1000 ng/ml (Moukhadder et al., 2017). Annual MRI R2 or R2* to evaluate LIC also contributes on early HCC prevention through strict iron overload monitoring and treatment (Taher et al., 2008).
3. Treatment
Few data exist on efficacy of HCC treatments in patients with thalassaemia. Treatment strategies according to stage of HCC and severity of liver disease used for the treatment of HCC in the general population (i.e. surgical resection, transarterial chemoembolisation (TACE), percutaneous radiofrequency ablation (RFA) and ethanol injection) have been used with success (Mancuso, 2010; Mancuso et al., 2006, 2005). However, the administration of sorafenib, a multi-kinase inhibitor indicated for advanced HCC did not yield adequate response in three thalassaemic patients with HCC in an Italian study (Restivo Pantalone et al., 2010). Further multi-centre prospective studies need to be conducted in order to clarify the effectiveness of the new immunomodulatory anti-cancer drugs in thalassaemic patients. Liver transplantation for the treatment of HCC in thalassaemic patients remains controversial. Two studies by Manusco et al. and Restivo Pantalone et al. have shown promising results, but larger studies are needed to clarify the role of liver transplantation in these patients (Mancuso, 2010; Restivo Pantalone et al., 2010).
Box
Summary.
References
- Ahmadi-Ghezeldasht S., Badiei Z., Sima H.R., Hedayati-Moghaddam M.R., et al. Distribution of Hepatitis C Virus Genotypes in Patients with Major β-Thalassemia in Mashhad, Northeast Iran. Middle East Journal of Digestive Diseases. [Online] 2018;10(1):35–39. Available from: doi: [PMC free article: PMC5903925] [PubMed: 29682246] [CrossRef]
- Allali S., de Montalembert M., Brousse V., Chalumeau M., et al. Management of iron overload in hemoglobinopathies. Transfusion Clinique Et Biologique: Journal De La Societe Francaise De Transfusion Sanguine. [Online] 2017;24(3):223–226. Available from: doi: [PubMed: 28673501] [CrossRef]
- Behzadifar M., Gorji H.A., Bragazzi N.L. The prevalence of hepatitis C virus infection in thalassemia patients in Iran from 2000 to 2017: a systematic review and meta-analysis. Archives of Virology. [Online] 2018;163(5):1131–1140. Available from: doi: [PubMed: 29411135] [CrossRef]
- Borgna-Pignatti C., Garani M.C., Forni G.L., Cappellini M.D., et al. Hepatocellular carcinoma in thalassaemia: an update of the Italian Registry. British Journal of Haematology. [Online] 2014;167(1):121–126. Available from: doi: [PubMed: 24992281] [CrossRef]
- Borgna-Pignatti C., Rugolotto S., Stefano P.D., Zhao H., et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. [Online] 2004;89(10):1187–1193. Available from: doi: [PubMed: 15477202] [CrossRef]
- Brittenham G.M. Iron-chelating therapy for transfusional iron overload. The New England Journal of Medicine. [Online] 2011;364(2):146–156. Available from: doi: [PMC free article: PMC3078566] [PubMed: 21226580] [CrossRef]
- Capai L., Charrel R., Falchi A. Hepatitis E in High-Income Countries: What Do We Know? And What Are the Knowledge Gaps? Viruses. [Online] 2018;10(6) Available from: doi: [PMC free article: PMC6024799] [PubMed: 29799485] [CrossRef]
- Cappellini M.D., Cohen A., Piga A., Bejaoui M., et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. [Online] 2006;107(9):3455–3462. Available from: doi: [PubMed: 16352812] [CrossRef]
- Chaston T.B., Matak P., Pourvali K., Srai S.K., et al. Hypoxia inhibits hepcidin expression in HuH7 hepatoma cells via decreased SMAD4 signaling. American Journal of Physiology - Cell Physiology. [Online] 2011;300(4):C888–C895. Available from: doi: [PMC free article: PMC3074628] [PubMed: 21289291] [CrossRef]
- Chirico V., Rigoli L., Lacquaniti A., Salpietro V., et al. Endocrinopathies, metabolic disorders, and iron overload in major and intermedia thalassemia: serum ferritin as diagnostic and predictive marker associated with liver and cardiac T2* MRI assessment. European Journal of Haematology. [Online] 2015;94(5):404–412. Available from: doi: [PubMed: 25200112] [CrossRef]
- Delicou S., Maragkos K., Tambaki M., Kountouras D., et al. Transient Elastography (TE) is a Useful Tool for Assessing the Response of Liver Iron Chelation in Sickle Cell Disease Patients. Mediterranean Journal of Hematology and Infectious Diseases. [Online] 2018;10(1) Available from: doi: [Accessed: 12 February 2021] [PMC free article: PMC6131104] [PubMed: 30210742] [CrossRef]
- Deugnier Y., Turlin B. Iron and hepatocellular carcinoma. Journal of Gastroenterology and Hepatology. [Online] 2001;16(5):491–494. Available from: doi: [PubMed: 11350542] [CrossRef]
- Deugnier Y., Turlin B., Ropert M., Cappellini M.D., et al. Improvement in liver pathology of patients with β-thalassemia treated with deferasirox for at least 3 years. Gastroenterology. [Online] 2011;141(4):1202–1211. 1211.e1-3. Available from: doi: [PubMed: 21741344] [CrossRef]
- Di Marco V., Bronte F., Cabibi D., Calvaruso V., et al. Noninvasive assessment of liver fibrosis in thalassaemia major patients by transient elastography (TE) - lack of interference by iron deposition. British Journal of Haematology. [Online] 2010a;148(3):476–479. Available from: doi: [PubMed: 19930183] [CrossRef]
- Di Marco V., Capra M., Angelucci E., Borgna-Pignatti C., et al. Italian Society for the Study of Thalassemia and Haemoglobinopathies; Italian Association for the Study of the Liver. Management of chronic viral hepatitis in patients with thalassemia: recommendations from an international panel. Blood. 2010b;116(16):2875–2883. [PubMed: 20551378]
- Dodd R.Y., Notari E.P., Stramer S.L. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. [Online] 2002;42(8):975–979. Available from: doi: [PubMed: 12385406] [CrossRef]
- EASL HBV Guidelines. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of Hepatology. [Online] 2017;67(2):370–398. Available from: doi: [PubMed: 28427875] [CrossRef]
- EASL HCC Guidelines. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of Hepatology. [Online] 2018;69(1):182–236. Available from: doi: [PubMed: 29628281] [CrossRef]
- EASL HCV Guidelines. EASL recommendations on treatment of hepatitis C: Final update of the series☆ Journal of Hepatology. [Online] 2020;73(5):1170–1218. Available from: doi: [PubMed: 32956768] [CrossRef]
- EASL HEV Guidelines. EASL Clinical Practice Guidelines on hepatitis E virus infection. Journal of Hepatology. [Online] 2018;68(6):1256–1271. Available from: doi: [PubMed: 29609832] [CrossRef]
- Ferraioli G., Lissandrin R., Tinelli C., Scudeller L., et al. Liver stiffness assessed by transient elastography in patients with β thalassaemia major. Annals of Hepatology. [Online] 2016;15(3):410–417. Available from: doi: [PubMed: 27049495] [CrossRef]
- Fragatou S., Tsourveloudis I., Manesis G. Incidence of hepatocellular carcinoma in a thalassemia unit. Hemoglobin. [Online] 2010;34(3):221–226. Available from: doi: [PubMed: 20524812] [CrossRef]
- Fraquelli M., Cassinerio E., Roghi A., Rigamonti C., et al. Transient elastography in the assessment of liver fibrosis in adult thalassemia patients. American Journal of Hematology. [Online] 2010;85(8):564–568. Available from: doi: [PubMed: 20658587] [CrossRef]
- Girelli D., Pasino M., Goodnough J.B., Nemeth E., et al. Reduced serum hepcidin levels in patients with chronic hepatitis C. Journal of Hepatology. [Online] 2009;51(5):845–852. Available from: doi: [PMC free article: PMC2761995] [PubMed: 19729219] [CrossRef]
- Hézode C., Colombo M., Bourlière M., Spengler U., et al. Elbasvir/Grazoprevir for Patients With Hepatitis C Virus Infection and Inherited Blood Disorders: A Phase III Study. Hepatology (Baltimore, Md.). [Online] 2017;66(3):736–745. Available from: doi: [PubMed: 28256747] [CrossRef]
- Jang T.-Y., Lin P.-C., Huang C.-I., Liao Y.-M., et al. Seroprevalence and clinical characteristics of viral hepatitis in transfusion-dependent thalassemia and hemophilia patients. PLoS ONE. [Online] 2017;12(6) Available from: doi: [Accessed: 12 February 2021] [PMC free article: PMC5466320] [PubMed: 28598970] [CrossRef]
- Kountouras D., Tsagarakis N.J., Fatourou E., Dalagiorgos E., et al. Liver disease in adult transfusion-dependent beta-thalassaemic patients: investigating the role of iron overload and chronic HCV infection. Liver International: Official Journal of the International Association for the Study of the Liver. [Online] 2013;33(3):420–427. Available from: doi: [PubMed: 23402611] [CrossRef]
- Kowdley K.V. Hematologic side effects of interferon and ribavirin therapy. Journal of Clinical Gastroenterology. [Online] 2005;39(1) Suppl:S3–8. Available from: doi: [PubMed: 15597025] [CrossRef]
- Krittayaphong R., Viprakasit V., Saiviroonporn P., Wangworatrakul W., et al. Serum ferritin in the diagnosis of cardiac and liver iron overload in thalassaemia patients real-world practice: a multicentre study - Krittayaphong - 2018 - British Journal of Haematology - Wiley Online Library. British Journal of Haematology. [Online] 2018;182(2):301–305. Available from: doi: https://doi
.org/10.1111/bjh.14776. [PubMed: 28543061] - Maggio A., D’Amico G., Morabito A., Capra M., et al. Deferiprone versus deferoxamine in patients with thalassemia major: a randomized clinical trial. Blood Cells, Molecules & Diseases. [Online] 2002;28(2):196–208. Available from: doi: [PubMed: 12064916] [CrossRef]
- Maggio A., Filosa A., Vitrano A., Aloj G., et al. Iron chelation therapy in thalassemia major: a systematic review with meta-analyses of 1520 patients included on randomized clinical trials. Blood Cells, Molecules & Diseases. [Online] 2011;47(3):166–175. Available from: doi: [PubMed: 21843958] [CrossRef]
- Maira D., Cassinerio E., Marcon A., Mancarella M., et al. Progression of liver fibrosis can be controlled by adequate chelation in transfusion-dependent thalassemia (TDT). Annals of Hematology. [Online] 2017;96(11):1931–1936. Available from: doi: [PubMed: 28875336] [CrossRef]
- Mancuso A. Hepatocellular carcinoma in thalassemia: A critical review. World Journal of Hepatology. [Online] 2010;2(5):171–174. Available from: doi: [PMC free article: PMC2999281] [PubMed: 21160991] [CrossRef]
- Mancuso A., Rigano P., Renda D., Di Salvo V., et al. Hepatocellular carcinoma on cirrhosis-free liver in a HCV-infected thalassemic. American Journal of Hematology. 2005;78(2):158–159. [PubMed: 15682406]
- Mancuso A., Sciarrino E., Renda M.C., Maggio A. A prospective study of hepatocellular carcinoma incidence in thalassemia. Hemoglobin. [Online] 2006;30(1):119–124. Available from: doi: [PubMed: 16540424] [CrossRef]
- Mangia A., Sarli R., Gamberini R., Piga A., et al. Randomised clinical trial: sofosbuvir and ledipasvir in patients with transfusion-dependent thalassaemia and HCV genotype 1 or 4 infection. Alimentary Pharmacology & Therapeutics. [Online] 2017;46(4):424–431. Available from: doi: [PubMed: 28660640] [CrossRef]
- Mavrogeni S., Kolovou G., Bigalke B., Rigopoulos A., et al. Transplantation in patients with iron overload: is there a place for magnetic resonance imaging? : Transplantation in iron overload. Heart Failure Reviews. [Online] 2018;23(2):173–180. Available from: doi: [PubMed: 29359261] [CrossRef]
- Moukhadder H.M., Halawi R., Cappellini M.D., Taher A.T. Hepatocellular carcinoma as an emerging morbidity in the thalassemia syndromes: A comprehensive review. Cancer. [Online] 2017;123(5):751–758. Available from: doi: [PubMed: 27911488] [CrossRef]
- Ou G., Ko H.H., Tiwari P., Sandhu N., et al. Utility of Transient Elastography in Estimating Hepatic Iron Concentration in Comparison to Magnetic Resonance Imaging in Patients Who are Transfusion-Dependent: A Canadian Center Experience. Hemoglobin. [Online] 2017;41(1):21–25. Available from: doi: [PubMed: 28532285] [CrossRef]
- Papadopoulos N., Kountouras D., Malagari K., Tampaki M., et al. Characteristics and Prognosis of Hepatocellular Carcinoma in Multi-Transfused Patients with β-Thalassemia. Experience of a Single Tertiary Center. Mediterranean Journal of Hematology and Infectious Diseases. [Online] 2020;12(1):e2020013. Available from: doi: [PMC free article: PMC7059739] [PubMed: 32180908] [CrossRef]
- Paparo F., Cevasco L., Zefiro D., Biscaldi E., et al. Diagnostic value of real-time elastography in the assessment of hepatic fibrosis in patients with liver iron overload. European Journal of Radiology. [Online] 2013;82(12):e755–761. Available from: doi: [PubMed: 24050879] [CrossRef]
- Philippe M.A., Ruddell R.G., Ramm G.A. Role of iron in hepatic fibrosis: One piece in the puzzle. World Journal of Gastroenterology : WJG. [Online] 2007;13(35):4746–4754. Available from: doi: [PMC free article: PMC4611196] [PubMed: 17729396] [CrossRef]
- Pipaliya N., Solanke D., Parikh P., Ingle M., et al. Comparison of Tissue Elastography With Magnetic Resonance Imaging T2* and Serum Ferritin Quantification in Detecting Liver Iron Overload in Patients With Thalassemia Major. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. [Online] 2017;15(2):292–298.e1. Available from: doi: [PubMed: 27650324] [CrossRef]
- Politis C., Zervou E., Drosou M., Siourounis P., et al. The First Documented Case of Acute Transfusion Transmitted Hepatitis E (TT-HEV) in Thalassaemia. Vox Sanguinis. 2018;113 S1:447.
- Poustchi H., Eslami M., Ostovaneh M.R., Modabbernia A., et al. Transient elastography in hepatitis C virus-infected patients with beta-thalassemia for assessment of fibrosis. Hepatology Research: The Official Journal of the Japan Society of Hepatology. [Online] 2013;43(12):1276–1283. Available from: doi: [PubMed: 23489382] [CrossRef]
- Procopet B., Berzigotti A. Diagnosis of cirrhosis and portal hypertension: imaging, non-invasive markers of fibrosis and liver biopsy. Gastroenterology Report. [Online] 2017;5(2):79–89. Available from: doi: [PMC free article: PMC5421457] [PubMed: 28533906] [CrossRef]
- Puliyel M., Sposto R., Berdoukas V.A., Hofstra T.C., et al. Ferritin trends do not predict changes in total body iron in patients with transfusional iron overload. American Journal of Hematology. [Online] 2014;89(4):391–394. Available from: doi: [PubMed: 24347294] [CrossRef]
- Refaai M.A., Blumberg N. Transfusion immunomodulation from a clinical perspective: an update. Expert Review of Hematology. [Online] 2013;6(6):653–663. Available from: doi: [PubMed: 24168641] [CrossRef]
- Restivo Pantalone G., Renda D., Valenza F., D’Amato F., et al. Hepatocellular carcinoma in patients with thalassaemia syndromes: clinical characteristics and outcome in a long term single centre experience. British Journal of Haematology. [Online] 2010;150(2):245–247. Available from: doi: [PubMed: 20433678] [CrossRef]
- Sharifi Z., Milani S., Shooshtari M.M. Study on Efficacy of Hepatitis B Immunization in Vaccinated Beta-thalassemia Children in Tehran. Iranian Journal of Pediatrics. 2010;20(2):211–215. [PMC free article: PMC3446027] [PubMed: 23056706]
- Sikorska K., Bernat A., Wroblewska A. Molecular pathogenesis and clinical consequences of iron overload in liver cirrhosis. Hepatobiliary & pancreatic diseases international: HBPD INT. [Online] 2016;15(5):461–479. Available from: doi: [PubMed: 27733315] [CrossRef]
- Sinakos E., Kountouras D., Koskinas J., Zachou K., et al. Treatment of chronic hepatitis C with direct-acting antivirals in patients with β-thalassaemia major and advanced liver disease. British Journal of Haematology. [Online] 2017;178(1):130–136. Available from: doi: [PubMed: 28439915] [CrossRef]
- Sousos N., Sinakos E., Klonizakis P., Adamidou D., et al. Deferasirox improves liver fibrosis in beta-thalassaemia major patients. A five-year longitudinal study from a single thalassaemia centre. British Journal of Haematology. [Online] 2018;181(1):140–142. Available from: doi: [PubMed: 28106901] [CrossRef]
- Taher A., El-Beshlawy A., Elalfy M.S., Al Zir K., et al. Efficacy and safety of deferasirox, an oral iron chelator, in heavily iron-overloaded patients with beta-thalassaemia: the ESCALATOR study. European Journal of Haematology. [Online] 2009;82(6):458–465. Available from: doi: [PMC free article: PMC2730551] [PubMed: 19187278] [CrossRef]
- Taher A., Rassi F.E., Isma’eel H., Koussa S., et al. Correlation of liver iron concentration determined by R2 magnetic resonance imaging with serum ferritin in patients with thalassemia intermedia. Haematologica. [Online] 2008;93(10):1584–1586. Available from: doi: [PubMed: 18728025] [CrossRef]
- Triantos C., Kourakli A., Kalafateli M., Giannakopoulou D., et al. Hepatitis C in patients with β-thalassemia major. A single-centre experience. Annals of Hematology. [Online] 2013;92(6):739–746. Available from: doi: [PubMed: 23412560] [CrossRef]
- University of Liverpool. Liverpool HEP Interactions. [Online]. 2021. Interaction Query Service; 2021. Available from: https:
//hep-druginteractions.org/query [Accessed: 2 March 2021]
- Review Evaluation of Liver Disease in Pregnancy.[Clin Liver Dis. 2023]Review Evaluation of Liver Disease in Pregnancy.Karim G, Giri D, Kushner T, Reau N. Clin Liver Dis. 2023 Feb; 27(1):133-155. Epub 2022 Oct 18.
- Review Effect of Korean Red Ginseng in chronic liver disease.[J Ginseng Res. 2017]Review Effect of Korean Red Ginseng in chronic liver disease.Park TY, Hong M, Sung H, Kim S, Suk KT. J Ginseng Res. 2017 Oct; 41(4):450-455. Epub 2017 Jan 5.
- Review Comparison between metabolic-associated fatty liver disease and nonalcoholic fatty liver disease: From nomenclature to clinical outcomes.[World J Hepatol. 2023]Review Comparison between metabolic-associated fatty liver disease and nonalcoholic fatty liver disease: From nomenclature to clinical outcomes.Alomari M, Rashid MU, Chadalavada P, Ragheb J, Zafar H, Suarez ZK, Khazaaleh S, Gonzalez AJ, Castro FJ. World J Hepatol. 2023 Apr 27; 15(4):477-496.
- Review Redefining non-alcoholic fatty liver disease to metabolic associated fatty liver disease: Is this plausible?[World J Hepatol. 2022]Review Redefining non-alcoholic fatty liver disease to metabolic associated fatty liver disease: Is this plausible?Devi J, Raees A, Butt AS. World J Hepatol. 2022 Jan 27; 14(1):158-167.
- [Ten years of research progress in the field of non-viral liver disease].[Zhonghua Gan Zang Bing Za Zhi....][Ten years of research progress in the field of non-viral liver disease].Wang QX, Ma X. Zhonghua Gan Zang Bing Za Zhi. 2021 Feb 20; 29(2):116-120.
- Liver disease - 2021 GuidelinesLiver disease - 2021 Guidelines
Your browsing activity is empty.
Activity recording is turned off.
See more...