NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cappellini MD, Farmakis D, Porter J, et al., editors. 2021 Guidelines: For the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 4th edition. Nicosia (Cyprus): Thalassaemia International Federation; 2023.

2021 Guidelines: For the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 4th edition.
Show detailsIron overload occurs when iron intake is increased over a sustained period of time, either as a result of red blood cell transfusions or increased absorption of iron through the gastrointestinal (GI) tract. Both of these occur in thalassaemias, with blood transfusion therapy being the major cause of iron overload in thalassaemia major and increased GI absorption being more important in non-transfusion dependent thalassaemia (NTDT). When thalassaemia major patients receive regular blood transfusion, iron overload is inevitable because the human body lacks a mechanism to excrete excess iron. Iron accumulation is toxic to many tissues, causing heart failure, cirrhosis, liver cancer, growth retardation and multiple endocrine abnormalities.
Chelation therapy aims to balance the rate of iron accumulation from blood transfusion by increasing iron excretion in urine and /or faeces with chelators. If chelation has been delayed or has been inadequate, it will be necessary to excrete iron at a rate that exceeds this. Because iron is also required for essential physiological purposes, a key challenge of chelation therapy is to balance the benefits of chelation therapy with the unwanted effects of excessive chelation. Careful dose adjustment is necessary to avoid excess chelation as iron levels fall. The second major challenge in chelation therapy is to achieve regular adherence to treatment regimens throughout life, as even short periods of treatment interruption can have damaging effects. While the convenience and tolerability of individual chelators is important in achieving this goal, other factors such as psychological well being and family and institutional support also impact on adherence and outcomes.
The Rate of Iron Loading
Blood transfusion
Gaining the most accurate information on the rate of iron loading from transfusion therapy is important in assisting selection of the best chelation therapy for each patient. A unit processed from 420 ml of donor blood contains approximately 200 mg of iron, or 0.47 mg/ml of whole donor blood. For red cell preparations with variable haematocrits, the iron in mg/ml of blood can therefore be estimated from 1.16 x the haematocrit of the transfused blood product. In cases where organisational systems or other difficulties prevent such estimations from being calculated, a rough approximation can be made based on the assumption that 200 mg of iron is contained in each donor unit. Irrespective of whether the blood used is packed, semi-packed or diluted in additive solution, if the whole unit is given, this will approximate to 200 mg of iron intake. According to the recommended transfusion scheme for thalassaemia major, the equivalent of 100–200 ml of pure red blood cell (RBC) per kg body weight per year are transfused. This is equivalent to 116-232 mg of iron/kg body weight/year, or 0.32-0.64 mg/kg/day. Regular blood transfusion therapy therefore increases iron stores to many times the norm unless chelation treatment is provided. If chelation therapy is not given, Table 1 shows how iron will accumulate in the body each year, or each day.
Table 1
Iron loading rates in the absence of chelation.
Increased gastrointestinal absorption of iron
In transfusion-dependent thalassaemia (TDT), the contribution of iron absorbed from the diet is small compared with blood transfusion. Normal intestinal iron absorption is about 1-2 mg/day. In patients with thalassaemia who do not receive any transfusion, iron absorption increases several-fold. It has been estimated that iron absorption exceeds iron loss when expansion of red cell precursors in the bone marrow exceeds five times that of healthy individuals. Transfusion regimens aimed at keeping the pre-transfusion haemoglobin above 90 g/l have been shown to prevent such expansion (Cazzola et al., 1997). In individuals who are poorly transfused, absorption rises to 3-5 mg/day or more, representing an additional 1-2 g of iron loading per year.
Toxicity from Iron Overload
Mechanisms of iron toxicity
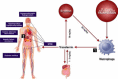
Iron is highly reactive and easily alternates between two states – iron3+ and iron2+ – in a process which results in the gain and loss of electrons, and the generation of harmful free radicals (atoms or molecules with unpaired electrons). These can damage lipid membranes, organelles and DNA, causing cell death and the generation of fibrosis. In health, iron is ‘kept safe’ by binding to molecules such as transferrin, but in iron overload the capacity to bind iron is exceeded both within cells and in the plasma compartment. The resulting ‘free iron’, either within cells or in plasma, damages many tissues in the body or is fatal unless treated by iron chelation therapy. Free iron also increases the risk of infections and neoplasia.
Distribution and consequences of transfusional iron overload
In the absence of iron overload, uptake of iron into cells is controlled by the interaction of transferrin with its receptors – mainly on red cell precursors, hepatocytes and dividing cells. In iron overload, transferrin becomes saturated and iron species that are not bound to transferrin are present in plasma (plasma non-transferrin bound iron, or NTBI). The distribution of NTBI uptake is fundamentally different from transferrin uptake, and is thought to involve calcium channels. Organ damage in transfusional iron overload reflects the pattern of tissue iron uptake from NTBI. Some tissue are spared from iron loading through this mechanism (such as skeletal muscle), while others such myocardial muscle, endocrine tissue and hepatocytes take up NTBI rapidly. This iron is then stored as ferritin or haemosiderin which are visible by magnetic resonance imaging (MRI). The myocardial iron overload can induce heart failure from cardiomyopathy in patients without chelation in as early as the second decade of life. Iron overload also causes pituitary damage, leading to hypogonadism, growth retardation and delayed puberty.
Endocrine complications, namely diabetes mellitus, hypothyroidism and hypoparathyroidism are also seen. Liver disease with fibrosis and eventually cirrhosis and hepatocellular carcinoma, particularly if concomitant chronic viral hepatitis is present, are also serious complications.
Monitoring of Iron Overload
Monitoring is essential in establishing effective iron chelation regimes, tailored to individuals’ specific needs. However, some general principles of monitoring iron overload apply to all.
Serum ferritin
Why measure serum ferritin?
Serum ferritin (SF) generally correlates with body iron stores, and is relatively easy and inexpensive to determine repeatedly. Serum ferritin is most useful in identifying trends. A decreasing trend in SF is good evidence of decreasing body iron burden but absence of a decreasing trend does not exclude a decreasing iron burden. However, an increasing SF trend implies an increasing iron burden but may also be due to inflammation or tissue damage, so clinical judgment must be used to interpret these trends.
Long term control of SF is also a useful guide to the risk of complications from iron overload in thalassaemia major (TM); many studies have shown an association between the control of serum ferritin and prognosis (Borgna-Pignatti et al., 2004; Davis et al., 2004; Gabutti & Piga, 1996; Olivieri et al., 1994). Studies have identified a significantly lower risk of cardiac disease and death in at least two-thirds of cases where serum ferritin levels have been maintained below 2,500 μg/l (with deferoxamine – desferrioxamine, DFO) over a period of a decade or more (Olivieri et al., 1994). Observations with larger patient numbers show that maintenance of an even lower serum ferritin of 1,000 μg/l may be associated with additional clinical advantages (Borgna-Pignatti et al., 2004) (see Table 2).
Table 2
Use of serum ferritin for monitoring chelation treatment.
What are the limitations of serum ferritin measurements?
Most SF assays were developed mainly for detecting iron deficiency, and the linear range of the assay at high SF values needs to be known. SF must be performed in a laboratory that has established how to dilute samples with high values, to give readings within the linear range of the assay. SF measures do not always predict body iron or trends in body iron accurately. In TM, variation in body iron stores accounts for only 57% of the variability in serum ferritin (Brittenham et al., 1993). This variability is in part because inflammation increases serum ferritin, and partly because the distribution of liver iron between macrophages (Kupffer cells) and hepatocytes in the liver has a major impact on serum ferritin. A sudden increase in serum ferritin should prompt a search for hepatitis, other infections, or inflammatory conditions. A lack of fall in SF with chelation does not therefore necessarily prove that the patient is a ‘non responder’ to the chelation regime.
As outlined above, this can be because inflammation may have falsely raised SF, or because the relationship between body iron and SF is not always linear, particularly in the context of inflammation or tissue damage (Adamkiewicz et al., 2009), and body iron can fall considerably from a high starting point (e.g. liver iron concentration >30 mg/g dry weight) before a change in ferritin is clear. Below 3000 μg/l SF values are influenced mainly by iron stores in the macrophage system, whereas above 3000 μg/l they are determined increasingly by ferritin leakage from hepatocytes (Davis et al., 2004; Worwood et al., 1980). Day-to-day variations are particularly marked at these levels. The relationship between serum ferritin and body iron stores may also vary depending on the chelator used (Ang et al., 2010) and by duration of chelation therapy (Fischer et al., 2003).
Why monitoring liver iron concentration?
- To identify whether body iron is adequately controlled.
Adequate control of LIC is linked to the risk of hepatic damage as well as the risk of extrahepatic damage. Normal LIC values are up to 1.8 mg/g dry weight (wt), with levels of up to 7 mg/g dry wt seen in some non-thalassaemic populations without apparent adverse effects. Sustained high LIC (above 15-20 mg/g dry wt) have been lined to worsening prognosis, liver fibrosis progression (Angelucci et al., 1997) or liver function abnormalities (Jensen et al., 2003). In the absence of prior iron chelation therapy, the risk of myocardial iron loading increases with the number of blood units transfused and hence with iron overload (Jensen et al., 2003; Buja & Roberts, 1971). However, the relationship between LIC and extra-hepatic iron is complicated by chelation therapy as iron tends to be accumulate initially in the liver and later in the heart but also is removed more rapidly from the liver than the heart by chelation therapy (Noetzli et al., 2008; Anderson et al., 2004). Thus, in patients receiving chelation therapy, whilst high LIC increases the risk of cardiac iron overload, the measurement of LIC will not predict myocardial iron and hence cardiac risk reliably, and myocardial iron may be found in some patients despite currently well controlled LIC.
- To determine iron balance: is body iron increasing or decreasing on current therapy?
LIC is the most reliable indicator of body iron load, which can be derived from the following formula: Total body iron stores in mg iron /kg body wt = 10.6 x the LIC (in mg/g dry wt) (Angelucci et al., 2000). Sequential measurement of LIC is the best way to determine whether body iron is increasing or decreasing with time (iron balance). While serum ferritin is simple, relatively inexpensive and can be repeated frequently, LIC determination should be considered for those patients whose serum ferritin levels deviate from expected trends (i.e. those with suspected co-existing hepatitis, or patients on chelation regimens with variable or uncertain responses), as this may reduce the risk of giving either inadequate or excessive doses of chelation therapy. Since the relationship of SF to iron overload and iron balance has not yet been established, assessment of LIC may be particularly useful when new chelating regimes are being used. At high levels of SF (>4000 μg/l), the relationship to LIC is not linear and patients may show a fall in LIC (negative iron balance) without a clear trend in SF in the first 6-12 months. When a patient fails to show a fall in SF over several months the change in LIC can identify whether the current regime is adequate or need to be modified (increased frequency or adherence, increased dose or change in regime).
Methods for measuring LIC
- Biopsy
Measurement of LIC was initially done by chemical determination on a liver biopsy sample (fresh, fixed or from dewaxing of paraffin-embedded material) (see Table 3). Biopsy is an invasive procedure, but in experienced hands has a low complication rate (Angelucci et al., 1997). Inadequate sample size (less than 4 mg dry wt or less than about a 2.5 cm core length) or uneven distribution of iron, particularly in the presence of cirrhosis (Villeneuve et al., 1996), may give misleading results however. Biopsy also allows the evaluation of liver histology which cannot yet be reliably estimated by non-invasive means. Laboratory standardisation is not trivial and differences between laboratories, for example in wet to dry weight ratios, can mean that results from different laboratories may not be equivalent.
Table 3
Rationale, advantages and disadvantages of (LIC) determination by (MRI) and biopsy.
- SQUID
Magnetic biosusceptometry (SQUID) (superconducting quantum interference device) determines the paramagnetism of the liver which is proportional to LIC (Brittenham et al., 1993). Current methodology requires liquid helium which is very expensive. Furthermore, the SQUID apparatus needs to be in an environment away from paramagnetic forces (e.g. lifts, cars) which is often impractical. For these reasons, the current generation of SQUID devices are unlikely to be used outside a small number of well-resourced centres. Surprisingly not all SQUID devices have been calibrated in the same way, so comparison of results from different centres must be interpreted with caution unless the relevant machines have been cross-validated.
- MRI
MRI techniques are now becoming the most widely used methods for LIC determination. The first techniques compared the signal in the liver or heart with that of skeletal muscle, which does not accumulate iron (Jensen et al., 1994). However, this is not in widespread use today and has been superseded by better methods. The principle shared by all MRI techniques currently used is that when a radio-frequency (rf) magnetic field pulse is applied to the tissue (e.g. liver or myocardium), protons take up energy, altering their spin orientation and they later relax returning to their original state. With spin echo, after the pulse the nuclei take time to relax in the ’relaxation time’; T1 in the longitudinal plane, and T2 in the transverse plane. Values may also be expressed as relaxation rates, the R1 rate (the same as 1/T1) and the R2 rate (the same as 1/T2). A variation of this principle are Gradient Echo techniques, achieved by applying a strong graded magnetic field to the rf pulse that is used for spin echo. This relies on multiple echoes over a shorter acquisition time than spin echo techniques. The shorter acquisition time may improve sensitivity and can be measured as T2* (in ms), where 1/T2* = 1/T2 + 1/T2’, and T2 is the tissue relaxation time and T2’ is the magnetic inhomogeneity of the tissue. An important point is that tissue iron concentration is not linearly related to T2* or the T2, but is linearly related to 1/T2* or 1/T2 (R2* or R2). Both gradient and/or spin echo techniques have been used in clinical practice. T2* (or R2*) can be achieved with a single breath hold, while T2 or R2 take a little longer to acquire data. Manufacturers of suitable MRI scanners are: Siemens (Erlangen, Germany); GE Healthcare (Milwaukee, WI, USA); Philips Healthcare (Amsterdam Netherlands). The strength of the magnetic field applied by these scanners is measured in Tesla (T) units. Most imaging is done on 1.5T machines but 3T machines give a better signal to noise ratio. However, 3T machines have greater susceptibility to artefacts, and the maximum detectable iron level is also halved (which is too low for many patients) (Wood & Ghugre, 2008; Storey et al., 2007). At present only 1.5T machines are widely used with reliable precision and accuracy based on standardised validation procedures. Liver packages (including standard sequences and analysis of the data) are included in the software provided with these MRI machines. Specialised LIC analysis software can also be bought separately.
A note of caution is that the different MRI techniques may not be equivalent – at least in the manner they are currently calibrated and practiced. The first widely used technique was the T2* technique (Anderson et al., 2001), where liver biopsy was used to calibrate the method. Although this demonstrated the principle of T2* to measure liver iron, unfortunately due to factors such as long echo times (TE 2.2-20.1 ms), and multi breath-hold acquisition, the calibration differs from later techniques, and can underestimate LIC by two-fold. Therefore, studies using this calibration may underestimate LIC (Garbowski et al., 2009). The R2 based FerriScan® technique appears to have acceptable linearity and reproducibility up to LIC values of about 30 mg/g dry wt (St Pierre et al., 2005), with an average sensitivity of >85% and specificity of >92% up to an LIC of 15 mg/g dry wt, and has been registered in the EU and US. For calibration of FerriScan®, the MRI machine must use a Phantom supplied by the company, while the data acquired is sent via internet for analysis by dedicated FerriScan® software (payment per scan analysed). A particular advantage of this technique is that it can be applied with little training, at any centre with a reasonably up-to-date MRI machine (see Table 3).
Myocardial iron estimation: T2* and other tools
The physical principles of iron measurement for the heart by MRI are the same as for the liver (see above), with the additional challenge of measuring a moving object – the myocardium. The T2* (or R2*) techniques have the advantage over T2 or R2 in that they require shorter acquisition times and can be achieved with a single breathhold (Kirk et al., 2010). The utility of myocardial T2* (mT2*) MRI was originally identified on the basis of shortened T2* values <20 ms in patients with decreased left ventricular ejection fraction (LVEF) (Anderson et al., 2001). More recently the relationship between biochemically measured myocardial iron concentration and myocardial T2* has been shown using post-mortem myocardial material (Carpenter et al., 2011). Here, mean myocardial iron causing severe heart failure in 10 patients at post-mortem was 5.98 mg/g dry wt (ranging from 3.2 to 9.5 mg/g); levels that in the liver would not be regarded as harmful. The relationship of myocardial iron concentration (MIC) to T2* is: MIC (mg/g dry wt) = 45 * (T2* ms)-1.22 (Kirk et al., 2009). This relationship is non-linear so small changes in mT2* at values <10 ms may indicate relatively large changes in MIC. The risk of developing heart failure increases with T2* values <10 ms, which are associated with a 160 fold increased risk of heart failure in the next 12 months (Kirk et al., 2009). This risk further increases progressively with T2* values <10 ms, so that the proportion of patients developing heart failure in the next 12 months at T2* of 8-10 ms, 6-8 ms and <6 ms was 18%, 31%, and 52% respectively. These risks were derived from patients whose chelation therapy and adherence was not reported, so this risk may be less in patients taking regular chelation. For example, in a recent prospective study in patients with severe myocardial iron loading (T2* values <10 ms), no patients developed heart failure over a 2 years period while taking deferasirox (DFX) and deferoxamine (DFO) combination therapy (Aydinok et al., 2013).
In centres where the T2* method has been validated, the T2* value may have predictive value in identifying patients at high risk of developing deterioration in LVEF, thus allowing targeted intensification of treatment before heart failure develops. Prompt identification of patients at risk by MRI, timely therapeutic intervention and improved chelation options have contributed to decreasing number of cardiac deaths (Thomas et al., 2010; Voskaridou et al., 2019). T2* monitoring has now been established and validated internationally (Kirk et al., 2010), and is now recommended as part of yearly monitoring of multi-transfused patients at risk of developing myocardial iron loading. However, it is very important that a given centre undertakes procedures to independently validate and calibrate measurements of the method adopted, otherwise inappropriate assessment of heart failure prognosis may result. Table 4 summarizes advantages and disadvantages of using T2* MRI for monitoring cardiac iron overload.
Table 4
MRI T2* method to assess myocardial iron.
Cardiac function
Sequential monitoring of LVEF has been shown to identify patients at high risk of developing clinical heart failure (Davis et al., 2004; Davis, O’Sullivan & Porter, 2001). When LVEF fell below reference values, there was a 35-fold increased risk of clinical heart failure and death, with a median interval to progression of 3.5 years, allowing time for intensification of chelation therapy. This approach required a reproducible method for determination of LVEF (such as MUGA (Multigated Acquisition) scan or MRI), while echocardiography was generally too operator-dependent for this purpose. Furthermore, there is a clear need to identify high risk patients before there is a decline in LVEF. Myocardial T2* by MRI can achieve this and has additional predictive value (see above). However, as only a subset of patients with T2* values between 10 and 20 ms, or even with T2* less than 10 ms have abnormal heart function, sequential measurement of LVEF can identify the subset of patients who have developed decompensation of LV function and are therefore at exceptionally high risk and require very intensive chelation therapy (see below).
Monitoring of other organ function and iron-mediated damage
The monitoring of organ function as a marker of damage from iron overload is discussed more fully in other chapters. In general, by the time diabetes, hypothyroidism, hypoparathyroidism or hypogonadotropic hypogonadism (HH) have been identified, irreversible damage has set in and the focus then becomes replacing hormones. These are late effects and the primary aim of chelation therapy is to prevent such damage. Iron overloaded patients should be monitored for evidence of HH (growth and sexual development and biochemical markers of HH), diabetes mellitus (yearly oral glucose tolerance test (OGTT)), hypothyroidism and hypoparathyroidism. There has been recent interest in using MRI as a way of identifying iron-mediated damage to the endocrine system. Early work in this area showed good correlation between MRI findings (loss of pituitary volume) and biochemical markers of pituitary damage (Chatterjee et al., 1998). With improved MRI imaging, other endocrine organs have also been evaluated (Wood, 2007). It is of interest, that there is generally a close correlation between iron deposition in the heart and deposition in endocrine tissues, such as those of the pituitary and pancreas (Noetzli et al., 2009; Au et al., 2008). This supports the notion of shared uptake mechanisms for NTBI in heart and endocrine systems and supports clinical observations of shared risks in cardiac and endocrine systems once iron begins to escape from the liver.
24h Urinary iron estimation
Measurement of the urinary iron excretion has been used in assessing the effect on iron excretion by deferoxamine (about half of total iron excreted in urine) (Pippard, Callender & Finch, 1982) or deferiprone (over 80% of iron excreted in urine), but is not useful in patients treated with deferasirox, as nearly all the iron is excreted in faeces. Urine iron has also been used to compare effects of combination and monotherapy regimes containing deferiprone (DFP) (Aydinok et al., 2012; Mourad et al., 2003). The inherent variability in daily iron excretion necessitates repeated determinations and this is not widely used in routine monitoring
Plasma non-transferrin bound iron and labile plasma iron
As plasma iron that is not bound to transferrin (NTBI), is considered to be the main route through which iron is distributed to liver and extrahepatic targets of iron-overloaded thalassaemia major patients, levels of NTBI might be expected to correlate with the risk of damage to these tissues. Assays may estimate NTBI directly using a chelation capture method followed by high performance liquid chromatography (HPLC) (Singh, Hider & Porter, 1990) or by colorimetric analysis (Gosriwatana et al., 1999) or indirectly by exploiting the impact of labile iron species to oxidised fluorochrome, such as in the labile plasma iron (LPI) assay (Zanninelli, Breuer & Cabantchik, 2009; Cabantchik et al., 2005). A potential advantage of the LPI assay is that it is better suited to measurements when iron chelators are present in the plasma (Zanninelli, Breuer & Cabantchik, 2009). Whilst some loose associations of NTBI (Piga et al., 2009) or LPI (Wood et al., 2011) with some markers of cardiac iron or response to chelation have been found by some investigators, thus far measurements have not been sufficiently strongly predictive of cardiac risk to be recommended for routine clinical practice. This is partly because NTBI and LPI are highly labile, rapidly returning or even rebounding (Porter et al., 1996) after an iron chelator has been cleared (Zanninelli, Breuer & Cabantchik, 2009). Although NTBI correlates loosely with iron overload, it is affected by other factors such as ineffective erythropoiesis, the phase of transfusion cycle, and the rate of blood transfusion (Porter et al., 2011) adding to the complexity of interpreting levels (Hod et al., 2010). It is also not clear which methods identify the iron species that are most strongly inked to myocardial iron uptake. Therefore, although the measurement of NTBI (or LPI) has proved a useful tool for evaluating how chelators interact with plasma iron pools, its value as a guide to routine treatment or prognosis has yet to be clearly demonstrated.
Box
Summary, Recommendations and Grade of Evidence.
References
- Adamkiewicz T.V., Abboud M.R., Paley C., Olivieri N., et al. Serum ferritin level changes in children with sickle cell disease on chronic blood transfusion are nonlinear and are associated with iron load and liver injury. Blood. [Online] 2009;114(21):4632–4638. Available from: doi: [PMC free article: PMC2780299] [PubMed: 19721013] [CrossRef]
- Anderson L.J., Holden S., Davis B., Prescott E., et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. European Heart Journal. [Online] 2001;22(23):2171–2179. Available from: doi: [PubMed: 11913479] [CrossRef]
- Anderson L.J., Westwood M.A., Holden S., Davis B., et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. British Journal of Haematology. [Online] 2004;127(3):348–355. Available from: doi: https://doi
.org/10.1111/j .1365-2141.2004.05202.x. [PubMed: 15491298] - Ang A.L., Shah F.T., Davis B.A., Thomas A., et al. Deferiprone Is Associated with Lower Serum Ferritin (SF) Relative to Liver Iron Concentration (LIC) Than Deferoxamine and Deferasirox-Implications for Clinical Practice. Blood. [Online] 2010;116(21):4246–4246. Available from: doi: [CrossRef]
- Angelucci E., Brittenham G.M., McLaren C.E., Ripalti M., et al. Hepatic iron concentration and total body iron stores in thalassemia major. The New England Journal of Medicine. [Online] 2000;343(5):327–331. Available from: doi: [PubMed: 10922422] [CrossRef]
- Angelucci E., Giovagnoni A., Valeri G., Paci E., et al. Limitations of Magnetic Resonance Imaging in Measurement of Hepatic Iron. Blood. [Online] 1997;90(12):4736–4742. Available from: doi: [PubMed: 9389689] [CrossRef]
- Au W.-Y., Lam W.W.-M., Chu W.W.C., Yuen H.-L., et al. A cross-sectional magnetic resonance imaging assessment of organ specific hemosiderosis in 180 thalassemia major patients in Hong Kong. Haematologica. [Online] 2008;93(5):784–786. Available from: doi: [PubMed: 18450735] [CrossRef]
- Aydinok Y., Evans P., Manz C.Y., Porter J.B. Timed non-transferrin bound iron determinations probe the origin of chelatable iron pools during deferiprone regimens and predict chelation response. Haematologica. [Online] 2012;97(6):835–841. Available from: doi: [PMC free article: PMC3366647] [PubMed: 22180427] [CrossRef]
- Aydinok Y., Kattamis A., Cappellini M.D., El-Beshlawy A., et al. Deferasirox– Deferoxamine Combination Therapy Reduces Cardiac Iron With Rapid Liver Iron Removal In Patients With Severe Transfusional Iron Overload (HYPERION). Blood. [Online] 2013;122(21):2257–2257. Available from: doi: [CrossRef]
- Borgna-Pignatti C., Rugolotto S., Stefano P.D., Zhao H., et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. [Online] 2004;89(10):1187–1193. Available from: doi: [PubMed: 15477202] [CrossRef]
- Brittenham G.M., Cohen A.R., McLaren C.E., Martin M.B., et al. Hepatic iron stores and plasma ferritin concentration in patients with sickle cell anemia and thalassemia major. American Journal of Hematology. [Online] 1993;42(1):81–85. Available from: doi: [PubMed: 8416302] [CrossRef]
- Buja L.M., Roberts W.C. Iron in the heart. Etiology and clinical significance. The American Journal of Medicine. [Online] 1971;51(2):209–221. Available from: doi: [PubMed: 5095527] [CrossRef]
- Cabantchik Z.I., Breuer W., Zanninelli G., Cianciulli P. LPI-labile plasma iron in iron overload. Best Practice & Research. Clinical Haematology. [Online] 2005;18(2):277–287. Available from: doi: [PubMed: 15737890] [CrossRef]
- Carpenter J.-P., He T., Kirk P., Roughton M., et al. On T2* Magnetic Resonance and Cardiac Iron. Circulation. [Online] 2011;123(14):1519–1528. Available from: doi: [PMC free article: PMC3435874] [PubMed: 21444881] [CrossRef]
- Cazzola M., Borgna-Pignatti C., Locatelli F., Ponchio L., et al. A moderate transfusion regimen may reduce iron loading in beta-thalassemia major without producing excessive expansion of erythropoiesis. Transfusion. [Online] 1997;37(2):135–140. Available from: doi: [PubMed: 9051086] [CrossRef]
- Chatterjee R., Katz M., Oatridge A., Bydder G.M., et al. Selective loss of anterior pituitary volume with severe pituitary-gonadal insufficiency in poorly compliant male thalassemic patients with pubertal arrest. Annals of the New York Academy of Sciences. [Online] 1998;850:479–482. Available from: doi: [PubMed: 9668590] [CrossRef]
- Davis B., O’Sullivan C., Porter J. Value of LVEF monitoring in the long-term management of beta-thalassaemia. 8th International Conference on Thalassemia and the hemoglobinopathies (Athens); 2001. 2001 p.
- Davis B.A., O’Sullivan C., Jarritt P.H., Porter J.B. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. [Online] 2004;104(1):263–269. Available from: doi: [PubMed: 15001468] [CrossRef]
- Fischer R., Longo F., Nielsen P., Engelhardt R., et al. Monitoring long-term efficacy of iron chelation therapy by deferiprone and desferrioxamine in patients with β-thalassaemia major: application of SQUID biomagnetic liver susceptometry. British Journal of Haematology. [Online] 2003;121(6):938–948. Available from: doi: https://doi
.org/10.1046/j .1365-2141.2003.04297.x. [PubMed: 12786807] - Gabutti V., Piga A. Results of long-term iron-chelating therapy. Acta Haematologica. [Online] 1996;95(1):26–36. Available from: doi: [PubMed: 8604584] [CrossRef]
- Garbowski M.W., Carpenter J.-P., Smith G., Pennell D.J., et al. Calibration of Improved T2* Method for the Estimation of Liver Iron Concentration in Transfusional Iron Overload. Blood. [Online] 2009;114(22):2004–2004. Available from: doi: [CrossRef]
- Gosriwatana I., Loreal O., Lu S., Brissot P., et al. Quantification of non-transferrin-bound iron in the presence of unsaturated transferrin. Analytical Biochemistry. [Online] 1999;273(2):212–220. Available from: doi: [PubMed: 10469492] [CrossRef]
- Hod E.A., Zhang N., Sokol S.A., Wojczyk B.S., et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. [Online] 2010;115(21):4284–4292. Available from: doi: [PMC free article: PMC2879099] [PubMed: 20299509] [CrossRef]
- Jensen P.D., Jensen F.T., Christensen T., Eiskjaer H., et al. Evaluation of myocardial iron by magnetic resonance imaging during iron chelation therapy with deferrioxamine: indication of close relation between myocardial iron content and chelatable iron pool. Blood. [Online] 2003;101(11):4632–4639. Available from: doi: [PubMed: 12576333] [CrossRef]
- Jensen P.D., Jensen F.T., Christensen T., Ellegaard J. Non-invasive assessment of tissue iron overload in the liver by magnetic resonance imaging. British Journal of Haematology. [Online] 1994;87(1):171–184. Available from: doi: [PubMed: 7947241] [CrossRef]
- Kirk P., He T., Anderson L.J., Roughton M., et al. International Reproducibility of Single Breath-hold T2* Magnetic Resonance for Cardiac and Liver Iron Assessment among Five Thalassemia Centers. Journal of magnetic resonance imaging : JMRI. [Online] 2010;32(2):315–319. Available from: doi: [PMC free article: PMC2946327] [PubMed: 20677256] [CrossRef]
- Kirk P., Roughton M., Porter J., Walker J., et al. Cardiac T2* Magnetic Resonance for Prediction of Cardiac Complications in Thalassemia Major. Circulation. [Online] 2009;120(20):1961–1968. Available from: doi: [PMC free article: PMC2784198] [PubMed: 19801505] [CrossRef]
- Mourad F.H., Hoffbrand A.V., Sheikh-Taha M., Koussa S., et al. Comparison between desferrioxamine and combined therapy with desferrioxamine and deferiprone in iron overloaded thalassaemia patients. British Journal of Haematology. [Online] 2003;121(1):187–189. Available from: doi: [PubMed: 12670352] [CrossRef]
- Noetzli L.J., Carson S.M., Nord A.S., Coates T.D., et al. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. [Online] 2008;112(7):2973–2978. Available from: doi: [PMC free article: PMC2556627] [PubMed: 18650452] [CrossRef]
- Noetzli L.J., Papudesi J., Coates T.D., Wood J.C. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood. [Online] 2009;114(19):4021–4026. Available from: doi: [PMC free article: PMC2774543] [PubMed: 19726718] [CrossRef]
- Olivieri N.F., Nathan D.G., MacMillan J.H., Wayne A.S., et al. Survival in medically treated patients with homozygous beta-thalassemia. The New England Journal of Medicine. [Online] 1994;331(9):574–578. Available from: doi: [PubMed: 8047081] [CrossRef]
- Piga A., Longo F., Duca L., Roggero S., et al. High nontransferrin bound iron levels and heart disease in thalassemia major. American Journal of Hematology. [Online] 2009;84(1):29–33. Available from: doi: [PubMed: 19006228] [CrossRef]
- Pippard M.J., Callender S.T., Finch C.A. Ferrioxamine excretion in iron-loaded man. Blood. 1982;60(2):288–294. [PubMed: 7093519]
- Porter J.B., Abeysinghe R.D., Marshall L., Hider R.C., et al. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood. 1996;88(2):705–713. [PubMed: 8695819]
- Porter J.B., Lin K.-H., Beris P., Forni G.L., et al. Response of iron overload to deferasirox in rare transfusion-dependent anaemias: equivalent effects on serum ferritin and labile plasma iron for haemolytic or production anaemias. European Journal of Haematology. [Online] 2011;87(4):338–348. Available from: doi: [PMC free article: PMC3229702] [PubMed: 21649735] [CrossRef]
- Singh S., Hider R.C., Porter J.B. A direct method for quantification of non-transferrin-bound iron. Analytical Biochemistry. [Online] 1990;186(2):320–323. Available from: doi: [PubMed: 2363505] [CrossRef]
- St Pierre T.G., Clark P.R., Chua-anusorn W., Fleming A.J., et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. [Online] 2005;105(2):855–861. Available from: doi: [PubMed: 15256427] [CrossRef]
- Storey P., Thompson A.A., Carqueville C.L., Wood J.C., et al. R2* imaging of transfusional iron burden at 3T and comparison with 1.5T. Journal of magnetic resonance imaging: JMRI. [Online] 2007;25(3):540–547. Available from: doi: [PMC free article: PMC2884049] [PubMed: 17326089] [CrossRef]
- Thomas A.S., Garbowski M., Ang A.L., Shah F.T., et al. A Decade Follow-up of a Thalassemia Major (TM) Cohort Monitored by Cardiac Magnetic Resonance Imaging (CMR): Significant Reduction In Patients with Cardiac Iron and In Total Mortality. Blood. [Online] 2010;116(21):1011–1011. Available from: doi: [CrossRef]
- Villeneuve J.P., Bilodeau M., Lepage R., Côté J., et al. Variability in hepatic iron concentration measurement from needle-biopsy specimens. Journal of Hepatology. [Online] 1996;25(2):172–177. Available from: doi: [PubMed: 8878778] [CrossRef]
- Voskaridou E., Kattamis A., Fragodimitri C., Kourakli A., et al. National registry of hemoglobinopathies in Greece: updated demographics, current trends in affected births, and causes of mortality. Annals of Hematology. [Online] 2019;98(1):55–66. Available from: doi: [PubMed: 30196444] [CrossRef]
- Wood J.C. Diagnosis and management of transfusion iron overload: the role of imaging. American Journal of Hematology. [Online] 2007;82(12) Suppl:1132–1135. Available from: doi: [PMC free article: PMC2892928] [PubMed: 17963249] [CrossRef]
- Wood J.C., Ghugre N. Magnetic resonance imaging assessment of excess iron in thalassemia, sickle cell disease and other iron overload diseases. Hemoglobin. [Online] 2008;32(1–2):85–96. Available from: doi: [PMC free article: PMC2884397] [PubMed: 18274986] [CrossRef]
- Wood J.C., Glynos T., Thompson A., Giardina P., et al. Relationship between labile plasma iron, liver iron concentration and cardiac response in a deferasirox monotherapy trial. Haematologica. [Online] 2011;96(7):1055–1058. Available from: doi: [PMC free article: PMC3128226] [PubMed: 21393329] [CrossRef]
- Worwood M., Cragg S.J., Jacobs A., McLaren C., et al. Binding of serum ferritin to concanavalin A: patients with homozygous beta thalassaemia and transfusional iron overload. British Journal of Haematology. [Online] 1980;46(3):409–416. Available from: doi: [PubMed: 7448126] [CrossRef]
- Zanninelli G., Breuer W., Cabantchik Z.I. Daily labile plasma iron as an indicator of chelator activity in Thalassaemia major patients. British Journal of Haematology. [Online] 2009;147(5):744–751. Available from: doi: [PubMed: 19764989] [CrossRef]
- Review Pathophysiology and classification of iron overload diseases; update 2018.[Transfus Clin Biol. 2019]Review Pathophysiology and classification of iron overload diseases; update 2018.Brissot P, Troadec MB, Loréal O, Brissot E. Transfus Clin Biol. 2019 Feb; 26(1):80-88. Epub 2018 Aug 15.
- Review Recent advances in the pathophysiology, diagnosis and treatment of hereditary hemochromatosis and other iron overload syndromes.[Adv Clin Path. 2001]Review Recent advances in the pathophysiology, diagnosis and treatment of hereditary hemochromatosis and other iron overload syndromes.Pirisi M, Avellini C, Scott CA, Toniutto P, Intersimone D, Aprile G, Branca B, Fumo E. Adv Clin Path. 2001 Oct; 5(4):121-31.
- Review Secondary Iron Overload and the Liver: A Comprehensive Review.[J Clin Transl Hepatol. 2023]Review Secondary Iron Overload and the Liver: A Comprehensive Review.Pinyopornpanish K, Tantiworawit A, Leerapun A, Soontornpun A, Thongsawat S. J Clin Transl Hepatol. 2023 Aug 28; 11(4):932-941. Epub 2023 Feb 1.
- Review Cardiac complications in beta-thalassemia: From mice to men.[Exp Biol Med (Maywood). 2017]Review Cardiac complications in beta-thalassemia: From mice to men.Kumfu S, Fucharoen S, Chattipakorn SC, Chattipakorn N. Exp Biol Med (Maywood). 2017 Jun; 242(11):1126-1135. Epub 2017 May 9.
- Review Review article: the iron overload syndromes.[Aliment Pharmacol Ther. 2012]Review Review article: the iron overload syndromes.Siddique A, Kowdley KV. Aliment Pharmacol Ther. 2012 Apr; 35(8):876-93. Epub 2012 Mar 4.
- Iron overload: Pathophysiology, diagnosis and monitoring - 2021 GuidelinesIron overload: Pathophysiology, diagnosis and monitoring - 2021 Guidelines
Your browsing activity is empty.
Activity recording is turned off.
See more...