NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cappellini MD, Farmakis D, Porter J, et al., editors. 2021 Guidelines: For the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 4th edition. Nicosia (Cyprus): Thalassaemia International Federation; 2023.

2021 Guidelines: For the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 4th edition.
Show detailsIntroduction
The lifelong and multi-organ nature of transfusion-dependent thalassaemia is well reflected in the very content of this book. As patients advance in years, the basic needs in blood transfusion and iron chelation, even if and when provided appropriately and in accordance to international standards, gradually become inadequate to sustain life, maintain wellbeing, and achieve social integration. For this reason, specialists in several medical disciplines including but not confined to heart, liver and endocrine, are called upon to contribute by monitoring and offering proactive management of iron toxicity and organ dysfunction in their field of expertise (Angastiniotis & Eleftheriou, 2009). These considerations and needs lead to significant and multiple challenges in the organisation of integrated services so that the best possible conditions for patient care are achieved.
Historically, and based on TIF’s experience through its work globally, the need for establishing day transfusion centres for patients with transfusion-dependent thalassaemia (and other haemoglobin disorders), separate from the main haematology or paediatric wards, where they were ‘naturally’ admitted initially, arose first in areas and places where patients with these disorders were treated in sufficient numbers. This prompted policy-makers and healthcare professionals to recognise and respect that the needs of these patients are quite different from those of patients with malignant haematological conditions. So through the years, different arrangements (as seen in Table 1 below) were promoted with regards to providing more targeted, specialised services to patients with haemoglobin disorders.
Table 1
Within haematology departments: Development of RBC (non-malignant) clinics separate from malignant haematology services Dedicated space within paediatric units where patients remained well into their adolescence and in many cases into their adulthood
Provision of specialised care extended beyond the basic care of transfusion and iron chelation began to be recognised as an absolute necessity in those centres where:
- There was high disease(s) prevalence amongst the population whether in the indigenous (Cyprus, Greece, Italy) or in the well-established and integrated migrant population (e.g. UK, France);
- The scientific community and the national health competent authorities recognised the important disorders’ related medical, public health, social and economic repercussions, in the absence of any effective national policies for their prevention and appropriate management;
As a result, these disorders were prioritised on the country’s national health agenda and disease-specific policies and programmes under national coordination and support were developed, allowing patients to grow, age and have a good quality of life. The integration of specialised multidisciplinary care services into the management of patients with these disorders brought about the need for specialised, disease-specific training/education of the different medical specialties, and many and multiple organisational challenges to ensure appropriate coordination. Where such steps were taken and where these led to successful, meaningful integration of services, including active research activity, Reference or Expert Centres began to spring up.
Such centres first developed in the 1980s in Mediterranean countries including Cyprus, Greece and Italy, and subsequently in the UK and France in the latter more in the context of Rare Disease (RD) national strategies; however in all, at least initially, without any structured specific criteria. Both the knowledge and experience of what ‘optimal’ care was for these disorders as well as the research activity around the better understanding of their pathophysiology and medical needs were in those early days quite limited. The development of Reference/Expert Centres was guided almost solely by the needs of the growing, ageing patients; the services, patient care pathways and quality standards that these centres developed through the years became the core and solid basis for setting the criteria in later years for defining the role a Reference/Expert Centre for Haemoglobin Disorders should be fulfilling. Gradually these first centres were officially as well and not only by reputation, assigned by the country’s national competent authorities as Reference or Expert Centres undergoing regular and professional reviewing of their quality standards. Patients with haemoglobin disorders within and gradually from outside these countries began to be referred to these centres by their treating physicians for consultation, second opinion or for receiving specialised services that did not exist in their own area, region or country.
As national competent authorities and healthcare specialists across the world began to acknowledge the value of effectively addressing these disorders and to gather knowledge and experience at national level, clinics/centres of variable level of expertise, offering a different range of services of variable quality standards begun to spring up, particularly in the 1990s, in various countries across the world. In most of these however, mainly basic care was provided including blood transfusion and iron chelation, while Multidisciplinary Care (MDC), which is an essential element of the care of growing patients and a major component of a Reference/Expert centre, was and still is, largely lacking.
In most low and middle income countries, where more than 80% of the patients with these disorders live, the poor/weak medical and public health infrastructures (including haematology and transfusion services), the lack of universal health and social care coverage that is related mainly to their weak economies, and very importantly their focus on other health priorities, including communicable and common non-communicable diseases (NCDs) have not allowed such advances to take place.
In a few of these countries for example, even Non-Government Organisations (NGOs), including the Red Cross and patient/parent oriented ones, were encouraged by competent national authorities to contribute to the management of haemoglobin disorders. These have developed through the years in collaboration with medical and other healthcare professionals’ important services for patients, confined however only to the provision of transfusion services and iron chelation therapies. Certainly in such settings the provision of any extended specialised treatment is not possible. Therefore, patients need to be referred to hospital settings; in many instances in an uncoordinated manner and to medical specialists who (in the greatest majority) do not have specialised knowledge of the treatment of haemoglobin disorders.
The work of TIF for more than 35 years in over 60 countries across all regions of the world has exposed the naked truth: the development and integration of MDC services into the management of patients with these disorders and the promotion and establishment of Reference/Expert Centres are to-date components of care that are far from being adopted or implemented. Such advances can only happen if and when the basic, essential medical care in a centre or across a country has reached those quality levels that allows patients to grow satisfactorily, achieve social integration and have a reasonably acceptable, decent quality of life. This prerequisite can only be accomplished when the medical and public health infrastructures and quality standards are adequately strengthened in the context, as previously mentioned, of a healthcare system based on universal coverage. To-date such improvements are still to happen in most of the countries, and particularly in the developing ones where the majority of patients with these disorders live. The young age of the patients in these countries, who in their greatest majority do not reach ages beyond twenty or thirty years, confirms the fact that they are still receiving suboptimal basic care.
Bringing fragmented services together in an organised, collaborative manner and adopting international guidelines and standards of care is indeed very challenging, but it has been demonstrated beyond any doubt that such an effort maximises the patients’ benefits and facilitates their very cumbersome treatment pathways with timely interventions. Such an approach is reflected in the improvement of outcomes (Allen, Gillen & Rixson, 2009), encompassing both clinical and social outcomes that have already happened in some countries, while at the same time, and very importantly, leads to the creation of cost-effective services which are of benefit both to the healthcare system and public health. (Rocks et al., 2020).
Imperative to the success of multidisciplinary or interdisciplinary, integrated management of thalassaemia patients is the quality of the relationship between the haematology (or paediatric) team and the various supporting physicians across medical disciplines. The latter ones must be well organised, well-coordinated, interested in becoming involved, in acquiring experience in these conditions and very importantly they need to be supported and facilitated by the healthcare system of the country. Random consultations do not contribute to efficiency and effectiveness because accumulation of sufficient experience and knowledge requires a dedicated group of medical experts in the MDC, who will indeed be willing to receive education and better understanding of the management of haemoglobin disorders for those aspects that fall within their medical specialty.
In many centres the multidisciplinary approach to thalassaemia is even misunderstood as having a specialist to refer to once a complication has arisen. The whole concept of MDC and of a Reference Centre lies on the availability of proactive, quality and specialised interventions and in this context different medical specialists should be involved well before the appearance of complications i.e. regular involvement early in the patients’ life. In a multi-organ disease like thalassaemia there is no doubt as to the necessity and benefits of this approach and TIF has focused considerable attention on promoting this concept and on creating those tools and advisory groups that could support it.
Furthermore, it is more functional if all members of the MDC belong/have medical attachments to the same hospital. This is because meetings of the members of the MDC group must occur regularly, and also when an emergency decision for the health/condition of a patient needs to be taken. It is understood however that this may not be possible, but technology through teleconferences and telemedicine tools have given us valuable solutions for such challenges.
The role of the co-ordinator/leadership
In order to achieve effective functioning of the interdisciplinary team that could be reflected in the desired outcomes of early detection and reversal or minimisation of organ damage, the principles of teamwork and good communication must include first of all a competent team coordinator who is an expert physician in the clinical management of haemoglobin disorders. This should best be the lead physician (haematologist, paediatrician etc.) who is responsible for the routine, everyday care and who sees each patient most often. Mature leadership will ensure that scheduled visits to each specialist are implemented and that in turn different specialists communicate timely and comprehensively their results to allow the treating specialist to reach a decision on any treatment adjustments.
As the issue of communication is central to the functioning of the MDC team (Eleftheriou, 2017) ensuring its effectiveness is important and this can be achieved in a number of ways and through for example:
- Joint clinics
- Regular team meetings
- Case conferences
- Sharing of results, along with interpretation and discussion for joint decisions
- Using electronic disease-specific medical records with full access to all members of the team
Box
Involving patients in the discussions and decisions which concern their lives is critical and every effort should be made by all to establish a well-structured and regular programme of active and meaningful patients’ engagement.
Some of the key barriers therefore that may prevent the achievement of effectiveness of the work of the MDC include (i) lack of appropriate and structured coordination and efficient communication, (ii) lack or poor involvement of dedicated physicians across medical disciplines, (iii) inadequate implementation of common shared decisions (iv) lack of sufficient time given by the involved physicians to interact productively and discuss comprehensively the cases and (v) inadequate access to, or lack of existence of, well-informed patient records.
An example of the structure of interdisciplinary team for the care of haemoglobin disorders as extracted from some well-established European Reference Centres with successful patient outcomes is presented in the Table below (Table 2) (European Commission, 2016):
Interdisciplinary team for the care of haemoglobin disorders
Many other important heath care specialists are needed particularly when the centres are treating sickle cell disease (SCD) patients as well, which is the usual case in most centres across countries. Some of the key specialist services to which treating physicians and patients should have timely and well-co-ordinated access in this case are included in Table 3 below (which is by no means exhaustive):
Table 3
Erythrocytapheresis Pulmonary hypertension team
The place and role of the patient in the MDC of his/her condition
In the multidisciplinary team, one essential member, constituting the central component, is most frequently forgotten or his or her importance is under-recognised. This is the patient (or parent in the case of children). The patient’s involvement is important for the carer/patient relationship and in particular with regards to the need to support his/her self-management and for the continued patient concordance to prescribed treatment. The patient however, requires education, continued provision of reliable and up-to-date information along with strong encouragement and empowerment to take charge of his/her life under specialist guidance. Treatment planning should take into consideration, as far as possible, patient preferences, choices and lifestyle so that every effort to reach concordance is made. The active and meaningful engagement of patients is substantial in the better understanding of the patients’ needs, and thus in the better planning and more appropriate reforming of related policies. The aim for every government and treating physician is certainly to achieve good health and quality of life of their patients associated with a high level of social integration; and indeed this can only happen if and when the patient remains at the centre of decisions.
Reference or Experts’ Centres for Haemoglobin Disorders
Considerable work on this topic has been conducted mainly by the European Commission in the context of its work on promoting quality services for RDs across the EU. The many and complex challenges faced by patients/families and treating physicians in the early and accurate diagnosis, and management and monitoring of RDs are similar to those that characterise haemoglobin disorders, which in many countries are RDs. However, contrary to the many thousands of other RDs there is (and has existed for some time now) ample and reliable knowledge as well as experience for haemoglobin disorders with regards to early and accurate diagnosis, specialised monitoring, appropriate management and effective prevention.
The European Commission recognised RDs as a priority action area since the mid- 1990s and since then the different EU initiatives addressing RDs have predominantly focused on bringing together scattered resources and expertise across Member States. This is an effort that is certainly needed in the case of haemoglobin disorders as well – both across Europe, and more importantly across countries with developing economies where the majority of patients with these disorders live. In addition, EU initiatives and policies aimed to strengthen and empower research activities in order to provide more innovative drugs and therapies for RDs, as well as the development of national plans in every EU Member State to more effectively addressing the needs of RDs. Within this work, the European Commission established a special committee of experts, the EUCERD (European Union Committee of Experts on Rare Diseases), which focused on developing quality criteria for centres of expertise for RDs in Member States (2011) and recommendations on establishing RD European Networks (ERNs) (2013) (EUCERD, 2011). In this context, the idea of ERNs was integrated into an EU Directive (2011/24/EU) which is related to the application of EU patients’ rights in cross-border healthcare acknowledging that this is a major step towards more effectively promoting the sharing of knowledge/expertise and best practices and the creation of clearer structures and networks in the area of RDs by bringing together highly specialised providers across the EU (European Parliament, 2011).
Within the 24 ERNs that were established to cover 24 different RDs or families of RDs, aiming to share best practices for their care and cure, the ERN on haematological diseases (EuroBloodNet) is the one focused on rare blood disorders including haemoglobin disorders. Considerable work is being undertaken by this network to pool together knowledge and expertise from across the EU on these disorders (EuroBloodNet, 2021).
In the context of the above work of the EU, a number of relevant projects were launched including the European Network for Rare and Congenital Anaemias (ENERCA) which was relevant to haemoglobin disorders and other rare anaemias, and in which TIF was a major partner. Through this activity, TIF contributed along with other European and International medical and scientific experts in rare anaemias to the completion, amongst other important deliverables, of a book titled: “The Recommendations for Centres of Expertise in Rare Anemias: A White Book”(Corrons et al., 2014).This document reflects the extensive work that the EU has undertaken for over two decades for the benefit of rare anaemias including haemoglobin disorders, and indeed represents a major contribution towards the creation of a much needed European infrastructure of expertise around rare anaemias. The authors of this Chapter of the 4th edition of the Guidelines for the Management of Transfusion Dependent Thalassaemia, have felt that the above description was essential to be included, as the experience gained through the years by EU Member States in this field can be extended and constitute a sound basis for the countries outside and well beyond Europe to build upon.
Some of the benefits outlined below in Table 4 and in the context of the EU directive (2011/24/EU) (mentioned above) on facilitating and safeguarding the rights of patients with RDs, including rare anaemias and haemoglobin disorders, for obtaining cross-border healthcare, underscore the importance of developing and pooling together specialised knowledge and experience as well as of networking between centres of expertise. From these ideas and policies, if and when tailored to the needs and prevailing situation across any country or region of the world, patients with haemoglobin disorders, the healthcare specialists and the healthcare systems at large could benefit significantly, as has been the case with the RDs across the EU.
TIF, as a patient-driven umbrella organisation, has a constitutional mandate to continually identify ways and tools to promote the quality of care provided to patients with haemoglobin disorders (Angastiniotis & Eleftheriou, 2014). It has thus focused particular attention and considerable work on its educational programme since its establishment in 1986 and in this context TIF has initiated in 2017, a new project titled “TIF’s Certification Programme” focused on: the empowerment of national competent authorities, healthcare professionals and patient communities to dedicate work on promoting the MDC component and the establishment of Reference/Expert centres into their management strategy (Soteriades et al., 2017).
TIF’s vision through this project is to first identify and develop an extended list of centres/clinics within a country that treat patients with haemoglobin disorders, followed by an effort supported by TIF’s International Expert Advisors to ‘classify’ them based on set criteria (see below) including the range and quality standards of the services provided by each of them (TIF, 2017). The aim of TIF is to (i) identify those centres already qualified to perform the role of a Reference Centre today, (ii) to provide through its scientific advisors support to those treating centres that have the potential to upgrade their services and (iii) to support other treating centres to reach at least acceptable levels of quality basic care for their patients and (iv) very importantly to support the networking between them at national level and of the Reference/Expert Centres at Regional and international level.
The programme focuses on the application of specific quality standards for reference centres involved in the provision of care for patients with thalassaemia and other haemoglobin disorders. The TIF Quality Standards are based on the general principles already developed by the relevant organisations as outlined in Table 5 below:
The criterion for recognizing any centre as a reference/expert centre is certainly the quality of services and its patient-centred care, and not just availability of various technical components necessary for thalassaemia (and other haemoglobin disorders) care. It includes following national or international evidence-based guidelines, which allow for good patient outcomes.
A Reference / Expert Centres must, for example:
- Have the capacity to provide expert diagnosis of the disease as well as its long term complications
- Have the capacity to provide expert case management, based on best practice guidelines including a multidisciplinary approach and psychosocial support. These requirements imply experienced healthcare personnel in adequate numbers to ensure continuity of care
- Ensure that health care professionals work in a structured environment with clearly defined roles and hierarchy.
- Maintain a patient registry with the ability to report patient outcomes and other epidemiological information. Electronic information systems must be regarded as essential tools for the provision of quality services.
- Have regular auditing of clinical and laboratory guidelines
- Serve a sufficient number of patients (at least 50 transfusion-dependent-thalassaemia patients) to maintain staff experience. What is a sufficient number of patients is not clear but a consensus should be reached.
- Provide patients with sufficient knowledge and information to promote partnership models and self-management support
- Have a significant contribution to research as evidenced by peer reviewed publications
- Establish networking with
- Secondary treatment centres to provide education and share knowledge and expertise as well as expert opinion on challenging cases and
- Other centres of expertise nationally.
- Establish networks/collaborations with other Reference Centres outside the coun try – regional and international – to share best practices.
- Maintain close links with patient organisations and other community resources at national, regional and international level.
- Make a major contribution to educational activities
- Provide evidence of the improvement of patients’ survival, clinical outcomes and quality of life.
In addition, (i) there must be evidence of government and more specifically health system support, (ii) free access of patients to treatment modalities, (iii) the centres’ administrative structure, working hours and clinical space availability must also be taken into consideration, with the patient experience in mind, (iv) deficiencies and gaps must be promptly identified and corrected, (v) regularly assess the experience and knowledge of professional staff and (vi) the patient perceptions of the quality of the services and the relationship with the staff should be monitored regularly through professional tools and taken into account in quality assessment.
TIF Standards as described in TIF’s project for assessing the quality of the services provided to patients with haemoglobin disorders in the different domains comprising a Reference Centre are outlined in Tables 6 – 12 below:
Table 6
1. Governance.
Table 12
7. Information Management.
Table 7
2. Safety Concerns.
Table 8
3. Access to care.
Table 9
4. Partnership model.
Table 10
5. Guidelines and standards for clinical care.
Table 11
6. Quality improvement.
Through this Certification Programme, TIF may provide:
- On-site audit of the centre’s performance by external reviewers with regards to the quality of the processes, outcomes and structures involved in the care it provides to haemoglobinopathy patients.
- Technical support and recommendations for improvement to reach desired out comes.
- Networking opportunities with other regional and international centres for the exchange of knowledge and expertise.
Successful centres are granted the Certificate of TIF Collaborating Reference Centre for Haemoglobinopathies, and provided with needs-based technical support and personalised recommendations for continuous improvement in order to reach the desired outcomes.
The Certificate is valid for a period of 2 years before the evaluation team is called back to the centre to ensure that quality of care is maintained.
The key goals of this programme are to:
- Provide authoritative scientific opinions and advice on key topics in clinical management including accurate diagnostic techniques, blood safety, correct iron monitoring and dealing with complications through a multidisciplinary approach in order to achieve continuous improvement within the healthcare delivery system for haemoglobinopathies worldwide;
- Provide a mechanism for internal and external peer evaluation towards excellence and ensure each centre’s accountability for the service they provide to thalassaemia patients;
- Establish an international network of reference centres for the delivery of quality healthcare services for haemoglobinopathies worldwide;
- Improve patient and programme safety in all activities and initiatives;
- Facilitate all patients’ access to expert management and contribute to the reduction of inequalities in the care that patients receive;
- Provide educational and training outlets for the centre’s staff for stimulating the organization’s quality improvement efforts. Secondary centres will have the opportunity to send staff for training and continuing medical education to the certified centres;
- Provide networking opportunities with other regional and international treating centres and benefit from staff training and tele-consultation to enable stakeholders to promote quality services from central level (government) and
- Enhance community confidence in thalassaemia care in all affected countries.
Certainly, such upgrading of a centre’s services and quality standards requires considerable national collaboration, support and funding and this can only be achieved if and when its value to the patients, the healthcare professionals and the healthcare system itself are well recognised by the competent authorities; it must be emphasised that TIF’s close and official collaboration with the national authorities is a prerequisite for any activity related to this project.
This initial work of TIF cannot and is neither meant to replace the value of quality assessment tools, including simple but valuable ones, such as audit and peer review, already practiced in many countries mainly of the Western world. Moreover, it is certainly not meant to replace those dedicated accrediting organisations which offer their work at a cost to assess and establish quality standards in the services provided by health institutions – hospitals, clinics or centres, public or private.
Indeed, TIF strongly encourages competent authorities to adopt such a methodology where and when possible.
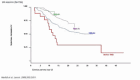
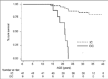
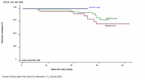
TIF through its programme outlined above, mainly aims to initiate an effort towards raising awareness on the value of MDC and Reference Centres in improving survival and quality of life of patients with these disorders, as has already been documented in a few countries (Figures 1-3). It aims to offer a simpler methodology as a first step to support the upgrading of services provided by treating centres particularly, but not only, of the developing economies by introducing the practice of MDC and by better acknowledging the value of the idea of pooling knowledge and experience and sharing best practices through the existence of Reference/Expert Centres.
Discussion and Conclusions
Patients globally are indeed faced with huge unmet needs both in basic medical care and to an even larger extent with regards to accessing MDC and expert review of their clinical status in Reference Centres with accumulated expertise. Both of these latter elements are unfortunately largely missing and according to TIF’s records, accumulated through its 35 years’ work at country level in over 60 countries across the world, such components are provided to less than 2% of the patients globally, constituting a severe violation of their rights both as humans and as patients.
It is hoped that the work of every country around the world towards promoting the UN Sustainable Development Goals 2030 (United Nations, 2021) and the work of the WHO on disease-specific but also many other relevant resolutions [WHO EB118. R1 on Thalassaemia & other Hemoglobinopathies (WHO, 2006b) and Resolution WHA59.20 on sickle cell anaemia (WHO, 2006a)], recommendations and programmes including blood and patient safety, will contribute towards achieving significant progress in the prevention and management of these disorders and will ‘allow’ them to further improve and introduce more specialised care, as described in this Chapter.
Healthcare services are becoming increasingly strained and healthcare authorities worldwide need to invest in integrated care particularly in the case of chronic, complex diseases such as the haemoglobin disorders, to first and above all deliver higher quality services for the patients while at the same time containing costs. Unfortunately, existing evidence of the cost-effectiveness of integrated care is limited particularly with regards to haemoglobin disorders. Future economic evaluation should target methodological issues to aid policy decisions with more robust evidence based on reliable, nationwide date (Aguilar Martinez et al., 2014).
It is also hoped that the contribution of this updated 4th edition of TIF’s Guidelines for the Management of Transfusion Dependent Thalassaemia, and the work of TIF at large, greatly supported by the WHO, the UN, the United Nations Economic and Social Council (ECOSOC), the EU, a large group of medical and scientific bodies and experts including the authors of this book, and very importantly by patients and families themselves, at national and international level, will contribute to the efforts of every country in providing a better future and ensuring more equity for patients with thalassaemia and other haemoglobin disorders.
References
- Aguilar Martinez P., Angastiniotis M., Eleftheriou A., Gulbis B., et al. Haemoglobinopathies in Europe: health & migration policy perspectives. Orphanet Journal of Rare Diseases. [Online] 2014;9:97. Available from: doi: [PMC free article: PMC4094755] [PubMed: 24980780] [CrossRef]
- Allen D., Gillen E., Rixson L. The Effectiveness of Integrated Care Pathways for Adults and Children in Health Care Settings: A Systematic Review. JBI library of systematic reviews. [Online] 2009;7(3):80–129. Available from: doi: [PubMed: 27820426] [CrossRef]
- Angastiniotis M., Eleftheriou A. Assessing services for haemoglobin disorders: A toolkit for service planning. [Online]. 2014. Available from: https://www
.researchgate .net/publication /297363633_Assessing _services_for_haemoglobin _disorders_A_toolkit _for_service_planning /citation/download [Accessed: 17 February 2021] - Angastiniotis M., Eleftheriou A. Requirements for a reference or expert thalassemia center: the structure/model for centers dealing with chronic/hereditary blood disorders. Hemoglobin. [Online] 2009;33 Suppl 1:S204–210. Available from: doi: [PubMed: 20001627] [CrossRef]
- Corrons J.-L.V., Pereira M., del M.M., Romeo-Casabona C., Nicolás P., et al. Recommendations for centres of expertise in rare anaemias. The ENERCA White Book. Thalassemia Reports. [Online] 2014;4(47878):86–90. Available from: doi: https://doi
.org/10.4081/thal.2014.4878. - Davis B., O’Sullivan C., Eliahoo J., Porter J. Survival in β-thalassaemia major: A single centre study. British Journal of Haematology-Supplement. 2001;113
- Eleftheriou P. Multidisciplinary care of Haemoglobinopathies: the UK example. 2017
- EUCERD. Recommendations on quality criteria for centres of expertise for rare diseases in Member States. [Online]. 2011. Available from: http://www
.eucerd.eu/?page_id=13. - EuroBloodNet. EuroBloodNet. [Online]. 2021. EuroBloodNet; 2021. Available from: https:
//eurobloodnet.eu/ [Accessed: 15 February 2021] - European Commission. European Reference Networks. [Online]. Public Health - European Commission; 2016. 25 November 2016. Available from: https://ec
.europa.eu/health/ern_en [Accessed: 15 February 2021] - European Parliament. Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross-border healthcare. Official Journal of the European Union. 2011;21
- Forni G.L., Puntoni M., Boeri E., Terenzani L., et al. The influence of treatment in specialized centers on survival of patients with thalassemia major. American Journal of Hematology. [Online] 2009;84(5):317–318. Available from: doi: [PubMed: 19396857] [CrossRef]
- Modell B., Khan M., Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet (London, England). [Online] 2000;355(9220):2051–2052. Available from: doi: [PubMed: 10885361] [CrossRef]
- Rocks S., Berntson D., Gil-Salmerón A., Kadu M., et al. Cost and effects of integrated care: a systematic literature review and meta-analysis. Eur J Health Econ. [Online] 2020;21(8):1211–1221. Available from: doi: [PMC free article: PMC7561551] [PubMed: 32632820] [CrossRef]
- Soteriades E.S., Andreas P., Angastiniotis M., Farmakis D., et al. Collaborating Reference Centres for Haemoglobinopathies Certification Programme. [Online]. 2017. Available from: https:
//thalassaemia .org.cy/wp-content/uploads /2018/06/Certification-Standards _FINAL.pdf. - TIF. TIF Certification Programme - TIF Collaborating Centres for Haemoglobinopathies. [Online]. 2017. TIF; 2017. Available from: https:
//thalassaemia .org.cy/projects/tif-certification-programme-2/ [Accessed: 15 February 2021] - United Nations. Sustainable Development Goals: 17 Goals to transform our world. [Online]. 2021. Sustainable Development Goals; 2021. Available from: https://www
.un.org/sustainabledevelopment /sustainable-development-goals/ [Accessed: 15 February 2021] - WHO. Sickle-cell Anaemia - Report by the Secretariat. [Online] 2006a:5. Available from: https://apps.who.int/iris/bitstream/handle/10665/20890/A59_9-en.pdf?sequence=1&is Allowed=y [Accessed: 15 February 2021]
- WHO. Thalassaemia and other haemoglobinopathies - Report by the Secretariat. [Online] 2006b:8. Available from: https://apps
.who.int /iris/bitstream/handle /10665/21519/B118_5-en .pdf?sequence=1&isAllowed=y [Accessed: 15 February 2021]
Footnotes
- 1
- Haemoglobin and red blood cell reference intervals during infancy.[Arch Dis Child. 2022]Haemoglobin and red blood cell reference intervals during infancy.Larsson SM, Hellström-Westas L, Hillarp A, Åkeson PK, Domellöf M, Askelöf U, Götherström C, Andersson O. Arch Dis Child. 2022 Apr; 107(4):351-358. Epub 2021 Oct 21.
- Review Reference centres for adults with rare and complex cancers - Policy recommendations to improve the organisation of care in Belgium.[Rev Epidemiol Sante Publique. ...]Review Reference centres for adults with rare and complex cancers - Policy recommendations to improve the organisation of care in Belgium.Stordeur S, Vrijens F, Leroy R. Rev Epidemiol Sante Publique. 2016 Feb; 64(1):1-6. Epub 2015 Dec 30.
- Audit of prenatal diagnosis for haemoglobin disorders in the United Kingdom: the first 20 years.[BMJ. 1997]Audit of prenatal diagnosis for haemoglobin disorders in the United Kingdom: the first 20 years.Modell B, Petrou M, Layton M, Varnavides L, Slater C, Ward RH, Rodeck C, Nicolaides K, Gibbons S, Fitches A, et al. BMJ. 1997 Sep 27; 315(7111):779-84.
- Evaluation of the accuracy of two point-of-care haemoglobin meters used in sub-Saharan African population: a cross-sectional study.[BMC Cardiovasc Disord. 2020]Evaluation of the accuracy of two point-of-care haemoglobin meters used in sub-Saharan African population: a cross-sectional study.Choukem SP, Sih C, Ntumsi AT, Dimala CA, Mboue-Djieka Y, Ngouadjeu EDT, Kengne AP. BMC Cardiovasc Disord. 2020 Mar 5; 20(1):111. Epub 2020 Mar 5.
- Review Effect of a multidisciplinary nutrition management model in patients with critical illness: A randomized trial.[Nurs Crit Care. 2024]Review Effect of a multidisciplinary nutrition management model in patients with critical illness: A randomized trial.Shunxia S, Jin Y, Xiaoling T, Juan H, Jiangqiong P. Nurs Crit Care. 2024 Mar; 29(2):417-426. Epub 2023 Nov 7.
- Multidisciplinary Care and Reference Centres in addressing haemoglobin disorders...Multidisciplinary Care and Reference Centres in addressing haemoglobin disorders - 2021 Guidelines
Your browsing activity is empty.
Activity recording is turned off.
See more...