NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cappellini MD, Farmakis D, Porter J, et al., editors. 2021 Guidelines: For the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 4th edition. Nicosia (Cyprus): Thalassaemia International Federation; 2023.

2021 Guidelines: For the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 4th edition.
Show detailsThe need for continuity of care and psychological support for chronic disease is widely accepted (Falvo, 2014; Lubkin & Larsen, 2013), as is the negative impact of psychological issues on chelation adherence (Beratis, 1989; Evangeli, Mughal & Porter, 2010; Panitz, 1999; Porter, Evangeli & El-Beshlawy, 2011) and the quality of life patients (Rikos et al., 2020) in thalassaemia major patients. This chapter will (1) provide a comprehensive review of the published social and behavioural problems in thalassaemia, with a specific focus on any suggested interventions, and (2) articulate the social and psychological support interventions that have been successfully used for similar problems in other diseases.
Unfortunately, there is a surprising lack of published evidence for psychological support interventions in thalassaemia. A 2001 Cochrane Review of psychological therapies for thalassaemia (Anie & Massaglia, 2001), assessed as “up-to-date” in 2014, concludes that “no randomised controlled trials employing psychological therapies … were identified” and “no trials, where quasi-randomization methods such as alteration are used, were found.” This is particularly concerning since a standard observation in many clinical reviews of thalassaemia over the past 25 years is that patient behaviour, primarily with adherence to iron chelation therapy (ICT), is a significant variable in long-term outcome and increased financial burden due to management of long term health complications (Borgna-Pignatti et al., 2004; Efthimiadis et al., 2006; Modell, Khan & Darlison, 2000; Olivieri et al., 1994; Porter & Davis, 2002; Vekeman et al., 2016). Non-adherence is episodic in many patients but over time probably just as damaging and further research is needed to see how this influences interventions (Vosper et al., 2018).
The Challenge of Psychological Support: What Does the Literature Tell Us?
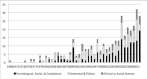
The challenge of ‘psychological support’ in thalassaemia is not a simple construct. Psychological support encompasses a complex set of defined responses to a diverse set of problems that have become apparent in thalassaemia over the past 30 years. This is illustrated by a simple PubMed Title/Abstract search for thalassaemia/thalassaemia and only “psychological support”. The first of eleven reports (including the Cochrane review) appears in 1985 identified the need for psychological support in a child care centre in Italy (Colombino & Bonzano, 1985), but it took over a decade before a second report described how psychosocial problems impacted chelation adherence, despite an expansion of clinical support services (Politis, 1998). This was restated in 2003 with a characterisation of adult patients (Galanello, 2003) and a cross sectional patient survey (Vardaki, Philalithis & Vlachonikolis, 2004). A small cluster of subsequent articles looked at “psychological burdens” in different patient groups including children and caregivers (Aydinok et al., 2005; Prasomsuk et al., 2007), adolescents (Roy & Chatterjee, 2007), and adults (Gharaibeh, Amarneh & Zamzam, 2009; Mednick et al., 2010). A single, non-randomised interventional study in 2009 used cognitive behavioural family therapy to try and alter adherence to chelation therapy (Mazzone et al., 2009). These results suggest a wide diversity in the application of psychological support in the clinical effort to manage the patient developmental pathway and their long-term survival associated with ICT adherence. This finding suggests that “psychological support” is an undefined response to a clinical need that requires specification. In order to develop a more complete understanding of the component elements of psychological support in thalassaemia, we conducted a comprehensive review of the 371 articles identified by a broad search of the “behavioural and social science research” (BSSR) literature (Figure 1). A full-text review determined that 9% (35) of the articles were either specific to BSSR or personal narratives. Another 11% (39) focused on clinical problems that happened to include a BSSR component (e.g. pregnancy in adult patients requires additional support services), and did not further an understanding of psychological support. The remaining articles are organised around the following clinical domains:
- Antenatal screening (30% of articles): these articles show a well-organised response to the problem of introducing antenatal screening in an at-risk population. They illustrate the complexity of creating a comprehensive solution that includes governmental support, legislation, community education, and face-to-face interaction. These reports tend to be post hoc celebrations of an arduous ad hoc process (TIF grade: D). The efforts to replicate this success have yielded some articles that identify specific complications associated with community demographic diversity in migrant populations. These articles identify the challenges this presents for implementing interventional strategies (TIF grade: C). Experience from antenatal screening that led to successful implementation were in relatively small and homogenous environments. However, the challenges when implementing clinical intervention in complex heterogeneous populations have not been fully considered. A few articles have addressed elements of this complex environment (Vichinsky et al., 2005) by looking at the economics of ICT (Payne et al., 2007; Riewpaiboon et al., 2010), clinical outreach to the communities of affected patients (Choy et al., 2000) and addressing the needs of culturally different patients (Banerjee et al., 2011) (TIF grade: C).
- Iron chelation therapy (10% of articles): most of these investigations either measure adherence (Matsui et al., 1994) or assess patient experience with treatment (Payne et al., 2007; Porter et al., 2012; Taher et al., 2010) (TIF grade: B). Over half of these articles appeared in the past 10 years with the introduction of new oral chelators and lay a scientific foundation for assessing the patient-reported health outcome as one step in understanding the patient’s ICT practices (Evangeli, Mughal & Porter, 2010; Mednick et al., 2010; Porter et al., 2012; Porter, Evangeli & El-Beshlawy, 2011; Sobota et al., 2011). These reports tend to have a very good scientific basis (TIF grade: A), because they are associated with other kinds of clinical investigations. They do not attempt to solve observed behavioural or social problems.
- Psychological problems (14% of articles): there appears to be a wide-ranging cross-national recognition that patients with thalassaemia are vulnerable to psychiatric problems (Aydinok et al., 2005; Cakaloz et al., 2009; Pradhan et al., 2003; Sadowski et al., 2002; Saini et al., 2007; Shaligram, Girimaji & Chaturvedi, 2007b, 2007a). These articles look at the psychological problems within the context of patient adherence to therapy, with the implied connection that failure to adhere reflects a patient’s psychological or cognitive makeup. The early reports tended to be at the level of clinical descriptive studies (TIF grade: C). More recent studies have shifted to identifying the neuropsychological investigation of cognitive deficits (Armstrong, 2005; Duman et al., 2011; Monastero et al., 2000; Zafeiriou, Economou & Athanasiou-Metaxa, 2006) (TIF grade: B). Angastiniotis points out that the problem of observed psychological problems in thalassaemia could actually be a function of the level and nature of support services that are available to patients (Angastiniotis, 2002) and not simply a problem of patient’s psychological makeup.
- Social support (20% of articles): these studies address the range of needs of families and patients. The effort to scientifically specify these needs began with Ratip’s work to develop disease-specific standardised assessments of these domains (Canatan et al., 2003; Ratip et al., 1995; Ratip & Modell, 1996) and has continued with other studies (Tsiantis et al., 1996; Zani, Di Palma & Vullo, 1995). This domain appears to have the most interventional studies that include targeting changes in institutional organisation practices (Marovic & Snyders, 2008), patient group sessions (Marovic & Snyders, 2008; Yamashita, Foote & Weissman, 1998), family therapy (Mazzone et al., 2009) and patient chelation camps (Treadwell & Weissman, 2001). While these reports suggest some success, they all lack a robust analytical assessment (TIF grade: C).
As a whole this literature suggests that patients with thalassaemia and their caregivers are faced with many distinct psychological and social challenges which have an impact on emotional functioning and may result in increased vulnerability to symptoms of psychiatric illnesses, such as depression and anxiety (Angastiniotis, 2002; Aydinok et al., 2005; Duman et al., 2011; Galanello, 2003; Gharaibeh, Amarneh & Zamzam, 2009; Marovic & Snyders, 2008; Politis, 1998; Prasomsuk et al., 2007; Ratip et al., 1995; Ratip & Modell, 1996; Roy & Chatterjee, 2007; Vardaki, Philalithis & Vlachonikolis, 2004; Zafeiriou, Economou & Athanasiou-Metaxa, 2006). Psychological support appears to be a loose reference to a broad mix of organisational responses to clinical needs, and not a coherent interventional strategy. Thus, there are no well-developed interventional trials aimed at providing psychological support to improve overall well-being of patients and their families (TIF grade: F). The few, small interventional studies are descriptive reports of clinic-level responses (TIF grade: C). They lack analytical rigor because standardised behavioural and social science research instruments were not used. Recent reports show an effort to develop the needed rigorous, scientific understanding of patient-reported outcomes within on-going studies of iron chelation therapy (Evangeli, Mughal & Porter, 2010; Haines et al., 2013; Porter et al., 2012; Porter, Evangeli & El-Beshlawy, 2011; Sobota et al., 2011; Trachtenberg et al., 2011, 2012b, 2012a). Most are designed to inform a clinical response to underlying clinical problems. These efforts should establish the analytical foundation for future interventional studies in psychological support.
In the meantime, we can only offer recommendations for psychological support based on existing best practices and research done with other disease populations.
Practical Considerations
Recommendations for standards of care for psychological support require a practical organisational model. As the specific challenges associated with being a patient with thalassaemia differ throughout development, a clinical pathway model that starts with the functional landmarks that define the patient and family experience is helpful (diagnosis-treatment). There are two modifiers to the clinical experience. Firstly, because thalassaemia is a chronic disease presenting shortly after birth, the natural growth from infant to adult will shape how patients learn to live with their disease. In the early stages, patients are dependent on their family caregivers, and as they develop, the patient must learn to successfully manage their own care. The second is the institutional organisation of clinical medicine. Paediatrics typically works with the patient and their family and adult medicine works with the individual patient. This situation complicates any psychological support recommendations. At each of the landmarks along the pathway (e.g. point of diagnosis, start of transfusion, initiation of chelation, transition into more self-care in adolescence, and transition to adult care), patients and families may be more vulnerable to experiencing psychological sequelae associated with the disease management and developmental challenges commonly experienced during that period of time. Our model of the ‘clinical pathway of thalassaemia’ is illustrated in figure 2.

Figure 2
Clinical pathway diagram.
Systematic studies to examine different intervention modalities that may help patients and families effectively cope with the particular challenges inherent at each time point are needed. These should address how early ‘upstream’ familial experiences impact ‘downstream’ patient adherence adaptations and long term survival. As most of the existing literature consists of descriptive reports and cross-sectional studies, the following practical recommendations are largely based on what we know from our clinical work and/or research with other chronic illnesses.
Point of diagnosis
Parents will undergo a series of changes after their child is diagnosed with thalassaemia (shock, denial, sadness/anger, adaptation, reorganisation) (Drotar et al., 1975). One of their most important immediate concerns is getting reliable information (Starke, 2002). Learning the additional tasks associated with caring for a child with thalassaemia can be overwhelming to the parent and lead to psychological distress (Galanello, 2003; Politis, 1998; Yamashita, Foote & Weissman, 1998). Importantly, if parents feel overwhelmed with caring for their child, effective management of the illness may become compromised (Otsuki et al., 2010). To minimise these feelings, effective psychological support of parents around the time of diagnosis should include:
Box
Providing necessary information about thalassaemia. This may need to be repeated several times for full comprehension. Opportunities to ask questions and share concerns.
It is especially important to help parents accept and learn to effectively cope with their child’s chronic medical condition at this early stage. This is because parental behaviours and attitudes throughout development will lay the groundwork for how children will cope with their condition. Parents who demonstrate healthy coping and understand that a well-managed patient who adheres to his/her therapy can live a successful life (Pakbaz et al., 2010) will help their children to learn to make thalassaemia a piece of who they are, rather than what defines them. Introducing the family to an appropriately experienced family with a child who has thalassaemia can be a helpful learning experience for parents of young children.
Start of blood transfusion
The best ways to provide psychological support aimed at helping children effectively cope with invasive medical procedures has been widely studied (Brown, 1999; Brown, Daly & Rickel, 2007; Edwards & Titman, 2011; Hayman, Mahon & PhD, 2002; Hymovich & Hagopian, 1992; Thompson, 2009). It is essential to help parents and children engage in effective coping strategies as soon as developmentally appropriate, as the experience of distress during a medical procedure has been found to be predictive of distress during future procedures (Frank et al., 1995).
Starting at a very young age, children often look to their parents for signals on how they should react in anxiety-provoking, novel situations. In one study, parent behaviour during an invasive medical procedure accounted for 53% of the variance in child distress behaviour (Frank et al., 1995). Providing information about the procedure prior to the actual procedure and giving the parent a job to do (e.g., distract the child), is likely to reduce parental anxiety, with positive indirect benefits for their children. However, if parents are not able to remain calm in front of their children during procedures such as blood transfusion, it is helpful for clinicians to give parents’ ‘permission’ to leave the room and instead consider including the presence of another supportive adult.
Specific coping strategies aimed directly at the child have been particularly useful in helping children cope effectively with invasive medical procedures. In a meta-analysis of psychological interventions for needle-related procedural distress in children and adolescents, distraction was found to be one of the most efficacious coping techniques (Uman et al., 2008). In fact, a recent study conducted with patients with thalassaemia found that bubble blowing during an injection helped reduce anxiety (Bagherain et al., 2012). Importantly, distraction techniques should be adapted to the child’s interest and age/developmental level. It is particularly useful to encourage parents who engage in excessive reassurance to instead focus on distracting their child, as reassurance often amplifies fear and distress (Manimala, Blount & Cohen, 2000), likely due to refocusing the child’s attention on the fearful and painful aspects of the situation.
As children get older, they may ask for more information about transfusions or other invasive medical procedures (e.g., magnetic resonance imaging). Fostering trust, reducing uncertainty, correcting misconceptions, enhancing the belief in their ability to cope with a procedure, and minimising distress are some of the potential benefits of providing advance information about a procedure to a child (Jaaniste, Hayes & Baeyer, 2007; Jipson & Melamed, 2007). Effective pre-procedural information should include:
In ideal clinical settings, a child-life specialist should be included on the treatment team. Child life specialists are health care professionals who have the specific training to understand the impact transfusion-dependent thalassaemia (TDT) has on children and can help them and their families navigate the complex processes needed to not only understand their treatment, but provide them with the necessary life-long skills not just to manage their clinical needs but to live with their disease.
Initiation of chelation
Parents need to be provided with support and guidance about choosing which type of chelation is best for their child. For example, although oral chelators are associated with less distress and better quality of life in older patients, due to specific developmental characteristics of very young children (e.g., transient food preference, oppositional behaviour, unpredictability), this may not be true for some children in this age group (Fiese, Wamboldt & Anbar, 2005). Parents of very young children need to be encouraged to carefully consider their chelation options, and determine which option best fits with their own capacities and their child’s personality characteristics. When starting chelation therapy, parents should be encouraged to develop consistent routines around medication taking. Developing predictable routines around a child’s medical regimen makes these tasks part of the typical daily schedule, thereby fostering good adherence by minimising several of the problems often associated with adherence difficulties (e.g., forgetting, conflicts about when to take the medication) (Fiese, Wamboldt & Anbar, 2005; Rand, 2005).
Behavioural interventions which include increased monitoring and incentives for meeting goals have been shown to be successful at improving adherence in patients with thalassaemia (Koch et al., 1993). The use of incentives may be particularly useful for paediatric patients who don’t yet understand the intrinsic value of adhering to an undesirable medical regimen. These may include verbal praise, stickers or small toys or other incentives earned either immediately or over time, for cooperating with daily chelation. By pairing a positive outcome (e.g., sticker, toy) with an aversive stimulus (chelation) the child develops a positive association with the aversive event, increasing the likelihood that the child will perform the behaviours again in the future.
At various times along the clinical pathway, patients may struggle with chelation adherence (Evangeli, Mughal & Porter, 2010). When this occurs, it is essential to identify why the patient is having difficulty following the prescribed plan. Interventions that do not consider the specific barrier to adherence will have limited success (see Table 1 for common barriers and suggested interventions). In general, effective interventions aimed at improving adherence usually:
Table 1
Common barriers to adherence and suggested interventions.
- Incorporate behavioural or multiple strategies;
- Include patients (and parents) in the development;
- Start from where the patient is at, gradually increasing goals, while working towards the ideal;
- Revise over time.
Older patients often make remarks such as “I am tired of doing this,” “I don’t want to,” or “I need a break.” Many often take the break. This ‘voluntary non-adherence’ is difficult to assess and many treating physicians do not often attempt to study this in their patients. It is important to continually monitor every patient’s adherence to ICT.
Additional opportunities for psychological support during childhood
As children with thalassaemia frequently miss school for medical appointments and transfusions (Gharaibeh, Amarneh & Zamzam, 2009), which can negatively impact school functioning (Thavorncharoensap et al., 2010), parents should be encouraged to educate the school about their child’s condition and to set-up plans which support the child when he/she needs to miss school. Further, patients with thalassaemia may be vulnerable to experiencing cognitive deficits (Armstrong, 2005; Duman et al., 2011; Economou et al., 2006; Lücke, Pfister & Dürken, 2005; Monastero et al., 2000; Nevruz et al., 2007; Zafeiriou, Economou & Athanasiou-Metaxa, 2006; Zafeiriou et al., 2004). If there are concerns from parents or the school, it may be valuable for patients to participate in neuropsychological testing to assess for any concerns and provide recommendations that could help support the patients learning potential.
Importance of social support throughout development
As social support has been found to play an important role in the psychological functioning of children and their families (Lewandowski & Drotar, 2007), starting from an early age, patients and their families would benefit from:
- Deciding how to present information about the patient’s medical condition to friends and family;
- Learning about the harmful effects (e.g., feelings of shame) of keeping thalassaemia a secret;
- Relying on existing friend, family, religious and community supports;
- Meeting other patients and families with chronic medical conditions through attending camps, events sponsored by specific illness foundations, or one-to-one meeting facilitated by a clinician.
Adolescence and transition to increased self-care
Adolescence is a time when adherence to daily medical regimens often declines (Trachtenberg et al., 2011). Frequently the transition of responsibility from the parent to the adolescent occurs before the patient is emotionally ready, resulting in poor adherence. Because adolescents are vulnerable to having their decision making driven by their desire to be independent and to fit-in with peers, parents need to continue to play an active role in monitoring adolescents self-care. Shared responsibility between the patient and caregiver has been found to be associated with better adherence (Evangeli, Mughal & Porter, 2010; Treadwell & Weissman, 2001).
Also, to avoid the negative consequences of abrupt shifts in responsibility, the transition of responsibility needs to:
- Occur gradually over time, starting when children are young (e.g., help gathering supplies) and increasing their involvement as they mature;
- Teach older patients how to take over responsibility for often-overlooked tasks, such as ordering supplies and making medical appointments.
Transition to adult care
One reason why adherence may be lowest in young adults (Trachtenberg et al., 2011) is because of insufficient psychosocial support as patients transition from paediatric to adult medical providers. Often the transition to adult care providers happens in an abrupt manner, leaving the patient unprepared for the shift to adult medicine (Bryant & Walsh, 2009). Discussions about transitions should occur well in advance of the actual transfer in care and should include an exploration of the patients concerns and how they will prepare for and manage the changes inherent in moving from a paediatric to an adult medicine clinic. This plan can result in fewer cases being ‘lost to follow-up’ (Allemang et al., 2019). A well-coordinated transition plan includes:
- Long-term plan that starts with orienting pre-adolescent patients for the change;
- Annual assessments of a patient’s preparation for transition;
- Multiple opportunities to orient the patient to an adult clinic, adult care practices, and the adult care system;
- Multiple overlapping visits with paediatric and adult haematologists.
Pain in thalassaemia patients
Pain is a concern for patients with thalassaemia. However, reports of pain in thalassaemia are relatively recent. Pain appears to increase in intensity and frequency with age (Haines et al., 2013; Trachtenberg et al., 2010). Because pain appears more frequently in older transfusion-dependent patients, pain in thalassaemia presents a new TDT problem.
The presence of pain usually indicates an underlying organic source, but in thalassaemia there is a lack of understanding of pain symptoms and its organic cause. The presence of pain in the non-thalassaemia adults is associated with decreased social function and increased depression (Avlund, Rantanen & Schroll, 2007; Burckhardt, 1985; Dunn & Croft, 2004; Garber et al., 2010; Koenig, 1997; Ozminkowski et al., 2012). For most patients who report the presence of pain, their response and choice of clinical intervention solutions is predicated on how they understand the source of the pain. If chronic pain symptoms are not modulated, patients are at risk of choosing non-clinically managed pharmacological solutions.
Because there is a lack of understanding of the relationship between thalassaemia pain symptoms and its organic cause, effective pain management strategies do not exist. If thalassaemia presents with pain symptoms, an effective treatment plan requires conducting a careful clinical evaluation to identify the underlying cause. Depending on the site of pain, there should be a consideration of clinical studies such as: bone density assessment, MRI/X-ray evaluation to assess musculoskeletal deformities or recent injuries, and an evaluation of average haemoglobin levels to elucidate the sources of pain (Lal, 2016; Piga, 2017). Based on these findings, a pain management strategy can be developed. Pain management plans can include pharmacological and nonpharmacological interventions; however, because most thalassaemia pain appears to be chronic, strong consideration should be given to nonpharmacological interventions such as:
Box
Physical Therapy Acupuncture/acupressure
Until the underlying organic studies of thalassaemia pain are conducted, clinicians need to routinely ask if the TDT patient has the presence of pain. If they report on having the presence of pain, then patient pain symptoms management strategies need to be routinely assessed. Clinicians should encourage patients with pain to engage in a variety of empirically validated (Eccleston et al., 2009; Palermo et al., 2010; Shega et al., 2012) cognitive and behavioural coping strategies which have been shown to successfully help patients manage their pain and distress through learning how to regulate their emotional and physical responses to pain.
Non-Adherence to Chelation Therapy
Patient adherence to their chelation therapy is the single-most important action that improves long-term outcome in TDT. Advances in chelation therapy have changed the expectation of patient long-term survival, and published studies suggest adherence rates as high as 90% (Pinto et al., 2018), 63% (Vekeman et al., 2016) and as low as 42% (Rofail et al., 2009). A recent Cochrane review of “Interventions for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia” (Fortin et al., 2018) suggests that the higher adherence reports with ICT could be an artefact of trial participation. Critically, the “quality of evidence” for adherence interventions was “low to very low across all” studies in the review, and only 3 trials “measured quality of life with validated instruments” and they “reported no difference in QoL” but “provided no analysable data.”
Regardless of any flaws in the published data, the introduction of new interventions has changed the life-expectancy for TDT patients. For patients who need life-saving chronic blood transfusions, it is incumbent on practitioners to be alert to changes in the patient’s ICT adherence practices. Change needs to be evaluated at several levels.
First, evaluation needs to start with measurable organic changes.
- Evaluating trends in iron loading: such as serial increases in ferritin levels in conjunction with increasing indications of organ involvement (using MRI reports).
- Other clinical tests that can be a useful indicator of iron burden: for example, measuring labile plasma iron (LPI), even though this is largely a research tool, can indicate iron burden (Steinberg-Shemer et al., 2018).
- Bioassays of chelator drug levels can provide an ‘objective’ measure of actual chelator use, but the tests are expensive for routine application
Second, patient reported adherence needs to be evaluated. Self–reports of adherence are inherently unreliable since patient subjective responses are highly variable and dependent on the patient-provider relationship. Most reported assessment methods are described in research studies but can be adjusted for clinical use:
- Basic standardised questionnaires have been devised to assess patient medication adherence. The most well-known is probably the Morisky Medication Adherence Scale (MMAS) (Moon et al., 2017).
- Pill counts or empty vial counts (in the case of deferoxamine) have been practiced with variable results. A refinement on this approach is to use the medication possession ratio (MPR), which is the proportion of days of medication supply a given drug in a particular time period, divided by the number of days in the time period (Sperber, Samarasinghe & Lomax, 2017).
- Medication Monitoring Devices have also been introduced. These include ‘digital pillboxes’ that have a ‘smart’ vial cap that electronically records the date and time of bottle opening. This is an expensive tool and studies have failed to show superiority to self-reporting questionnaires (Badawy et al., 2019; Monnette et al., 2018; Shi et al., 2010).
- Text messaging and mobile phone app interventions are another approach but there is a need to establish the long-term benefit.
Third, and most important, the patient needs to be engaged in developing solutions for their practices. A paternalistic (top down) approach, where the physician tells a patient that they have to do their therapy, will not work. As with parenting, where every parent knows that telling an adolescent or young adult what they have to do rarely yields the appropriate long-term action. With TDT, clinicians need to recognise that patients live their thalassaemia every day, many (especially older patients) know more about the disease burden than clinical practitioners. The lived experience frames their understanding and ultimately their actions. Finding effective long-term solutions requires building the patient/provider relationship. The relationship is built around trust and respect, allows for creative solutions aimed at helping a patient develop a coherent response to a long-term problem.
- Taking time to listen to concerns and provide support is the most essential action supporting patient adherence (Zolnierek & Dimatteo, 2009).
- Increasing patient knowledge and understanding of their condition results in patient empowerment which can encourage adherence to prescribed treatment (Náfrádi, Nakamoto & Schulz, 2017). As patients age, discussing laboratory results such as serum ferritin or MRI results, can help engage patients. These discussions not only enable patients to understand why the tests are needed, but they also inform them about their overall health trajectory.
- Utilising and trusting the input of the clinical team (other physicians, nurses and allied healthcare workers). The team gets to know the patient in different ways. Their insight into the social factors that shape adherence practice (illustrated, in part, in table 1) provide a more comprehensive under standing of the patient’s circumstance.
Whatever other method is used to engage patient adherence, it is important to remember that thalassaemia is no longer a ‘disease of childhood’”, it is a life long chronic disease. Patient adherence requires constant, periodic assessment. This lifelong relationship has negative side effects on the provider and the provider team. The amount of time required to build and sustain the relationship, can result in staff fatigue, burnout and information overload. Often, the time commitment to build and sustain the patient-provider relationship runs against the artificial limits imposed by the healthcare reimbursement system. Developing a workable solution requires a non-combative and collaborative ‘monitoring’ and ‘quality control’ approach by healthcare administrators (Wagner et al., 2001).
Psychosocial support throughout the lifespan as part of standard care
As social and emotional concerns can occur anywhere along the lifespan and such concerns can have an impact on the patient’s quality of life and physical health, opportunities for regular psychological support should be part of the treatment plan of all patients with thalassaemia. This is best accomplished through a comprehensive team approach used by thalassaemia centres of excellence found in high-income countries. The teams include multidisciplinary allied health specialists including skilled nursing staff, psychologists, social workers and other specialists such as family or child-life specialists. These health providers are better equipped to assess any social, emotional or cognitive concerns and intervene with additional support when necessary. If the team members regularly meet with patients and their families, it allows multiple communication pathways to be built. An effective team that is centred on improving patient response to therapy can take advantage of the multi-dimensional understanding of a patient, to create interventions that work for the patient. This is especially important when the team tries to build a novel adherence strategy for a patient. The different information pathways are also important for monitoring commonly seen anxiety and depression symptoms, and determining when those symptoms become psychiatric disorders that need early psychotherapy in order to prevent long-term health consequences. Importantly, the inclusion of psychological support as part of the standard care, some of the stigmatization associated with seeing a therapist may be removed.
Because TDT has become a chronic condition that begins at birth, there is a need for a comprehensive care framework that offers multidisciplinary care that spans both paediatrics and adult care. Because costs are a challenge, there are generally distinct choices of where national investments are made. Paediatric multidisciplinary teams are common in the US model of healthcare delivery for TDT. The US paediatric centres exert a significant level of energy to provide young patients with the tools they need to survive into adulthood. As young adults, they are then transitioned to systems where comprehensive care is not available where patients become ‘lost to follow up’. In the UK, healthcare resources are aimed at supporting the workforce. Multidisciplinary Teams are accessible for adults, but non-existent in paediatrics. The UK centres have to commit a high level of psychological resources aimed at providing the TDT patient with the necessary tools that allow them to learn to become a functional (working) adult. For many, learning the skills are often challenging, because they were not introduced to them at an earlier age. Efforts to bridge the UK and US models can be found, but finite resources usually require rationing access to multi-disciplinary centres of excellence, or by forcing a limit on the services provided by these ‘centres’. This situation is clearly suboptimal because thalassaemia is no longer a disease of childhood, but a clearly described life-long chronic condition that begins at birth. We need a new model for healthcare resource allocation and delivery to address these types of health problems.
Box
Summary, Recommendations and Grade of Evidence.
References
- Allemang B., Allan K., Johnson C., Cheong M., et al. Impact of a transition program with navigator on loss to follow-up, medication adherence, and appointment attendance in hemoglobinopathies. Pediatric Blood & Cancer. [Online] 2019;66(8):e27781. Available from: doi: [PubMed: 31045326] [CrossRef]
- Angastiniotis M. The adolescent thalassemic. The complicant rebel. Minerva Pediatrica. 2002;54(6):511–515. [PubMed: 12388938]
- Anie K.A., Massaglia P. Psychological therapies for thalassaemia. Cochrane Database of Systematic Reviews. [Online] 2001;(3) Available from: doi: [Accessed: 17 February 2021] [PubMed: 11687031] [CrossRef]
- Armstrong F.D. Thalassemia and learning: Neurocognitive functioning in children. Annals of the New York Academy of Sciences. [Online] 2005;1054:283–289. Available from: doi: [PubMed: 16339676] [CrossRef]
- Avlund K., Rantanen T., Schroll M. Factors underlying tiredness in older adults. Aging Clinical and Experimental Research. [Online] 2007;19(1):16–25. Available from: doi: [PubMed: 17332717] [CrossRef]
- Aydinok Y., Erermis S., Bukusoglu N., Yilmaz D., et al. Psychosocial implications of Thalassemia Major. Pediatrics International: Official Journal of the Japan Pediatric Society. [Online] 2005;47(1):84–89. Available from: doi: [PubMed: 15693873] [CrossRef]
- Badawy S.M., Morrone K., Thompson A., Palermo T.M. Computer and mobile technology interventions to promote medication adherence and disease management in people with thalassemia. The Cochrane Database of Systematic Reviews. [Online] 2019;6:CD012900. Available from: doi: [PMC free article: PMC6598413] [PubMed: 31250923] [CrossRef]
- Bagherain S., Borhani F., Abbaszadeh A., Ranjibar H., et al. The effect of distraction by bubble-making on the procedural anxiety of injection in Thalassemic school-age children in Kerman Thalasemia center. J. Nurs. Midwifery Q. -Shaheed Beheshti Univ. Med. Sci. Health Serv. 2012;22:52–59.
- Bandstra N.F., Skinner L., Leblanc C., Chambers C.T., et al. The role of child life in pediatric pain management: a survey of child life specialists. The Journal of Pain. [Online] 2008;9(4):320–329. Available from: doi: [PubMed: 18201933] [CrossRef]
- Banerjee A.T., Watt L., Gulati S., Sung L., et al. Cultural beliefs and coping strategies related to childhood cancer: the perceptions of South Asian immigrant parents in Canada. Journal of Pediatric Oncology Nursing: Official Journal of the Association of Pediatric Oncology Nurses. [Online] 2011;28(3):169–178. Available from: doi: [PubMed: 21646638] [CrossRef]
- Beratis S. Noncompliance with iron chelation therapy in patients with beta thalassaemia. Journal of Psychosomatic Research. [Online] 1989;33(6):739–745. Available from: doi: [PubMed: 2695624] [CrossRef]
- Bolig R., Yolton K.A., Nissen H.L. Medical play and preparation: questions and issues. Children’s Health Care: Journal of the Association for the Care of Children’s Health. [Online] 1991;20(4):225–229. Available from: doi: [PubMed: 10115571] [CrossRef]
- Borgna-Pignatti C., Rugolotto S., De Stefano P., Zhao H., et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187–1193. [PubMed: 15477202]
- Brown R.T. Cognitive Aspects of Chronic Illness in Children. Guilford Press; 1999. Google-Books-ID: BLImvNN37PcC.
- Brown R.T., Daly B.P., Rickel A.U. Chronic Illness in Children and Adolescents. Hogrefe Publishing; 2007. Google-Books-ID: rUFfAgAAQBAJ.
- Bryant R., Walsh T. Transition of the chronically ill youth with hemoglobinopathy to adult health care: an integrative review of the literature. Journal of Pediatric Health Care: Official Publication of National Association of Pediatric Nurse Associates & Practitioners. [Online] 2009;23(1):37–48. Available from: doi: [PubMed: 19103405] [CrossRef]
- Burckhardt C.S. The impact of arthritis on quality of life. Nursing Research. 1985;34(1):11–16. [PubMed: 3844156]
- Burns-Nader E.S. University of Alabama Libraries; 2011. The effects of medical play on reducing fear, anxiety, and procedure distress in school-aged children going to visit the doctor. thesis. [Online] Available from: http://ir
.ua.edu/handle/123456789/1301 [Accessed: 15 February 2021] - Cakaloz B., Cakaloz I., Polat A., Inan M., et al. Psychopathology in thalassemia major. Pediatrics International: Official Journal of the Japan Pediatric Society. [Online] 2009;51(6):825–828. Available from: doi: [PubMed: 19438833] [CrossRef]
- Canatan D., Ratip S., Kaptan S., Cosan R. Psychosocial burden of beta-thalassaemia major in Antalya, south Turkey. Social Science & Medicine (1982). [Online] 2003;56(4):815–819. Available from: doi: [PubMed: 12560014] [CrossRef]
- Choy J., Foote D., Bojanowski J., Yamashita R., et al. Outreach strategies for Southeast Asian communities: experience, practice, and suggestions for approaching Southeast Asian immigrant and refugee communities to provide thalassemia education and trait testing. Journal of Pediatric Hematology/Oncology. [Online] 2000;22(6):588–592. Available from: doi: [PubMed: 11132235] [CrossRef]
- Colombino G., Bonzano L. [Assistance for the thalassemic child in a child care center] Minerva Medica. 1985;76(6):209–211. [PubMed: 3974933]
- Drotar D., Baskiewicz A., Irvin N., Kennell J., et al. The adaptation of parents to the birth of an infant with a congenital malformation: a hypothetical model. Pediatrics. 1975;56(5):710–717. [PubMed: 1196728]
- Duman O., Arayici S., Fettahoglu C., Eryilmaz N., et al. Neurocognitive function in patients with β-thalassemia major. Pediatrics International: Official Journal of the Japan Pediatric Society. [Online] 2011;53(4):519–523. Available from: doi: [PubMed: 20964788] [CrossRef]
- Dunn K.M., Croft P.R. Epidemiology and natural history of low back pain. Europa Medicophysica. 2004;40(1):9–13. [PubMed: 16030488]
- Eccleston C., Palermo T.M., Williams A.C., de C., Lewandowski A., et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. The Cochrane Database of Systematic Reviews. [Online] 2009;(2):CD003968. Available from: doi: [PubMed: 19370592] [CrossRef]
- Economou M., Zafeiriou D.I., Kontopoulos E., Gompakis N., et al. Neurophysiologic and intellectual evaluation of beta-thalassemia patients. Brain & Development. [Online] 2006;28(1):14–18. Available from: doi: [PubMed: 15925466] [CrossRef]
- Edwards M., Titman P. Promoting Psychological Well-Being in Children with Acute and Chronic Illness. Jessica Kingsley Publishers; 2011. Google-Books-ID: oI9IVVgeUecC.
- Efthimiadis G.K., Hassapopoulou H.P., Tsikaderis D.D., Karvounis H.I., et al. Survival in thalassaemia major patients. Circulation Journal: Official Journal of the Japanese Circulation Society. [Online] 2006;70(8):1037–1042. Available from: doi: [PubMed: 16864938] [CrossRef]
- Evangeli M., Mughal K., Porter J.B. Which psychosocial factors are related to chelation adherence in thalassemia? A systematic review. Hemoglobin. [Online] 2010;34(3):305–321. Available from: doi: [PubMed: 20524820] [CrossRef]
- Falvo D.R. Medical and psychosocial aspects of chronic illness and disability. Fifth Edition. Burlington, MA: Jones & Bartlett Learning; 2014.
- Fiese B.H., Wamboldt F.S., Anbar R.D. Family asthma management routines: connections to medical adherence and quality of life. The Journal of Pediatrics. [Online] 2005;146(2):171–176. Available from: doi: [PubMed: 15689901] [CrossRef]
- Fortin P.M., Fisher S.A., Madgwick K.V., Trivella M., et al. Interventions for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia. The Cochrane Database of Systematic Reviews. [Online] 2018;5:CD012349. Available from: doi: [PMC free article: PMC5985157] [PubMed: 29737522] [CrossRef]
- Frank N.C., Blount R.L., Smith A.J., Manimala M.R., et al. Parent and staff behavior, previous child medical experience, and maternal anxiety as they relate to child procedural distress and coping. Journal of Pediatric Psychology. [Online] 1995;20(3):277–289. Available from: doi: [PubMed: 7595816] [CrossRef]
- Galanello R. A thalassemic child becomes adult. Reviews in Clinical and Experimental Hematology. 2003;7(1):4–21. [PubMed: 14692232]
- Garber C.E., Greaney M.L., Riebe D., Nigg C.R., et al. Physical and mental health-related correlates of physical function in community dwelling older adults: a cross sectional study. BMC geriatrics. [Online] 2010;10:6. Available from: doi: [PMC free article: PMC2835714] [PubMed: 20128902] [CrossRef]
- Gharaibeh H., Amarneh B.H., Zamzam S.Z. The psychological burden of patients with beta thalassemia major in Syria. Pediatrics International: Official Journal of the Japan Pediatric Society. [Online] 2009;51(5):630–636. Available from: doi: [PubMed: 19419527] [CrossRef]
- Haines D., Martin M., Carson S., Oliveros O., et al. Pain in thalassaemia: the effects of age on pain frequency and severity. British Journal of Haematology. [Online] 2013;160(5):680–687. Available from: doi: [PubMed: 23278768] [CrossRef]
- Hayman L.L., Mahon M.M., PhD J.R.T. Chronic Illness In Children: An Evidence-Based Approach. Springer Publishing Company; 2002. Google-Books-ID: 1UMFyRH8ytgC.
- Hymovich D.P., Hagopian G.A. Chronic illness in children and adults: A psychosocial approach. First. WB Saunders Company; 1992.
- Jaaniste T., Hayes B., Baeyer C.V. Providing Children With Information About Forth-coming Medical Procedures: A Review and Synthesis. Clinical Psychology : Science and Practice. [Online] 2007;14(2):124–143. Available from: doi: [CrossRef]
- Jipson J.L., Melamed B.G. New Approaches on the Horizon: Comments on Jaaniste, Hayes, and von Baeyer’s “Providing Children With Information About Forthcoming Medical Procedures: A Review and Synthesis” Clinical Psychology: Science and Practice. [Online] 2007;14(2):149–156. Available from: doi: https://doi
.org/10.1111/j .1468-2850.2007.00074.x. - Koch D.A., Giardina P.J., Ryan M., MacQueen M., et al. Behavioral contracting to improve adherence in patients with thalassemia. Journal of Pediatric Nursing. 1993;8(2):106–111. [PubMed: 8509968]
- Koenig H.G. Differences in psychosocial and health correlates of major and minor depression in medically ill older adults. Journal of the American Geriatrics Society. [Online] 1997;45(12):1487–1495. Available from: doi: [PubMed: 9400559] [CrossRef]
- Lal A. Assessment and treatment of pain in thalassemia. Annals of the New York Academy of Sciences. [Online] 2016;1368(1):65–72. Available from: doi: [PMC free article: PMC4870117] [PubMed: 27124110] [CrossRef]
- Lewandowski A., Drotar D. The relationship between parent-reported social support and adherence to medical treatment in families of adolescents with type 1 diabetes. Journal of Pediatric Psychology. [Online] 2007;32(4):427–436. Available from: doi: [PubMed: 17056638] [CrossRef]
- Lubkin I.M., Larsen P.D. Chronic illness: impact and intervention. Eighth ed. Burlington, Mass.: Jones & Bartlett Learning; 2013.
- Lücke T., Pfister S., Dürken M. Neurodevelopmental outcome and haematological course of a long-time survivor with homozygous alpha-thalassaemia: case report and review of the literature. Acta Paediatrica (Oslo, Norway: 1992). [Online] 2005;94(9):1330–1333. Available from: doi: [PubMed: 16279001] [CrossRef]
- Manimala M.R., Blount R.L., Cohen L.L. The effects of parental reassurance versus distraction on child distress and coping during immunizations. Children’s Health Care. [Online] 2000;29(3):161–177. Available from: doi: [CrossRef]
- Marovic S., Snyders F. Addressing Complexities of Medical Noncompliance in Serious Childhood Illness: Collaborating at the Interface of Providers, Families, and Health Care Systems. Families, Systems, & Health. [Online] 2008;26(3):237–249. Available from: doi: [CrossRef]
- Matsui D., Hermann C., Klein J., Berkovitch M., et al. Critical comparison of novel and existing methods of compliance assessment during a clinical trial of an oral iron chelator. Journal of Clinical Pharmacology. [Online] 1994;34(9):944–949. Available from: doi: [PubMed: 7983239] [CrossRef]
- Mazzone L., Battaglia L., Andreozzi F., Romeo M.A., et al. Emotional impact in beta-thalassaemia major children following cognitive-behavioural family therapy and quality of life of caregiving mothers. Clinical practice and epidemiology in mental health: CP & EMH. [Online] 2009;5:5. Available from: doi: [PMC free article: PMC2657903] [PubMed: 19236719] [CrossRef]
- McCue K. Medical Play: An Expanded Perspective. Children’s Health Care. [Online] 1988;16(3):157–161. Available from: doi: [CrossRef]
- Mednick L., Yu S., Trachtenberg F., Xu Y., et al. Symptoms of depression and anxiety in patients with thalassemia: Prevalence and correlates in the thalassemia longitudinal cohort. American journal of hematology. [Online] 2010;85(10):802–805. Available from: doi: [PMC free article: PMC4251654] [PubMed: 20806230] [CrossRef]
- Modell B., Khan M., Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet (London, England). [Online] 2000;355(9220):2051–2052. Available from: doi: [PubMed: 10885361] [CrossRef]
- Monastero R., Monastero G., Ciaccio C., Padovani A., et al. Cognitive deficits in beta-thalassemia major: Cognitive deficits in beta-thalassemia major. Acta Neurologica Scandinavica. [Online] 2000;102(3):162–168. Available from: doi: [PubMed: 10987375] [CrossRef]
- Monnette A., Zhang Y., Shao H., Shi L. Concordance of Adherence Measurement Using Self-Reported Adherence Questionnaires and Medication Monitoring Devices: An Updated Review. PharmacoEconomics. [Online] 2018;36(1):17–27. Available from: doi: [PubMed: 28895104] [CrossRef]
- Moon S.J., Lee W.-Y., Hwang J.S., Hong Y.P., et al. Accuracy of a screening tool for medication adherence: A systematic review and meta-analysis of the Morisky Medication Adherence Scale-8. PloS One. [Online] 2017;12(11):e0187139. Available from: doi: [PMC free article: PMC5667769] [PubMed: 29095870] [CrossRef]
- Náfrádi L., Nakamoto K., Schulz P.J. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PloS One. [Online] 2017;12(10):e0186458. Available from: doi: [PMC free article: PMC5645121] [PubMed: 29040335] [CrossRef]
- Nevruz O., Ulas U., Cetin T., Kutukcu Y., et al. Cognitive dysfunction in beta-thalassemia minor. American Journal of Hematology. [Online] 2007;82(3):203–207. Available from: doi: [PubMed: 17078021] [CrossRef]
- Olivieri N.F., Nathan D.G., MacMillan J.H., Wayne A.S., et al. Survival in medically treated patients with homozygous beta-thalassemia. The New England Journal of Medicine. [Online] 1994;331(9):574–578. Available from: doi: [PubMed: 8047081] [CrossRef]
- Otsuki M., Eakin M.N., Arceneaux L.L., Rand C.S., et al. Prospective Relationship between Maternal Depressive Symptoms and Asthma Morbidity among Inner-City African American Children. Journal of Pediatric Psychology. [Online] 2010;35(7):758–767. Available from: doi: [PMC free article: PMC2915621] [PubMed: 19850709] [CrossRef]
- Ozminkowski R.J., Musich S., Bottone F.G., Hawkins K., et al. The burden of depressive symptoms and various chronic conditions and health concerns on the quality of life among those with Medicare Supplement Insurance. International Journal of Geriatric Psychiatry. [Online] 2012;27(9):948–958. Available from: doi: [PubMed: 22025352] [CrossRef]
- Pakbaz Z., Treadwell M., Kim H.-Y., Trachtenberg F., et al. Education and Employment Status of Children and Adults with Thalassemia in North America. Pediatric blood & cancer. [Online] 2010;55(4):678–683. Available from: doi: [PMC free article: PMC2932798] [PubMed: 20535817] [CrossRef]
- Palermo T.M., Eccleston C., Lewandowski A., Williams A.C., de C., et al. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain. [Online] 2010;148(3):387–397. Available from: doi: [PMC free article: PMC2823996] [PubMed: 19910118] [CrossRef]
- Panitz D. Trauma and adolescence. International Universities Press, Inc; Madison, CT: 1999. Sugar M. Difficulties encountered by adolescent thalassemia patients.
- Payne K.A., Desrosiers M.-P., Caro J.J., Baladi J.-F., et al. Clinical and economic burden of infused iron chelation therapy in the United States. Transfusion. [Online] 2007;47(10):1820–1829. Available from: doi: [PubMed: 17880607] [CrossRef]
- Piga A. Impact of bone disease and pain in thalassemia. Hematology. American Society of Hematology. Education Program. [Online] 2017;2017(1):272–277. Available from: doi: [PMC free article: PMC6142535] [PubMed: 29222266] [CrossRef]
- Pinto V.M., Balocco M., Quintino S., Bacigalupo L., et al. Daily alternating deferasirox and deferiprone therapy successfully controls iron accumulation in untreatable transfusion-dependent thalassemia patients. American Journal of Hematology. [Online] 2018;93(10):E338–E340. Available from: doi: [PubMed: 30033633] [CrossRef]
- Politis C. The psychosocial impact of chronic illness. Annals of the New York Academy of Sciences. [Online] 1998;850:349–354. Available from: doi: [PubMed: 9668557] [CrossRef]
- Porter J., Bowden D.K., Economou M., Troncy J., et al. Health-Related Quality of Life, Treatment Satisfaction, Adherence and Persistence in β-Thalassemia and Myelodysplastic Syndrome Patients with Iron Overload Receiving Deferasirox: Results from the EPIC Clinical Trial. Anemia. [Online] 2012;2012:297641. Available from: doi: [PMC free article: PMC3424665] [PubMed: 22924125] [CrossRef]
- Porter J.B., Davis B.A. Monitoring chelation therapy to achieve optimal outcome in the treatment of thalassaemia. Best Practice & Research. Clinical Haematology. 2002;15(2):329–368. [PubMed: 12401311]
- Porter J.B., Evangeli M., El-Beshlawy A. Challenges of adherence and persistence with iron chelation therapy. International Journal of Hematology. [Online] 2011;94(5):453–460. Available from: doi: [PubMed: 21993873] [CrossRef]
- Pradhan P.V., Shah H., Rao P., Ashturkar D., et al. Psychopathology and self-esteem in chronic illness. Indian Journal of Pediatrics. [Online] 2003;70(2):135–138. Available from: doi: [PubMed: 12661807] [CrossRef]
- Prasomsuk S., Jetsrisuparp A., Ratanasiri T., Ratanasiri A. Lived experiences of mothers caring for children with thalassemia major in Thailand. Journal for specialists in pediatric nursing: JSPN. [Online] 2007;12(1):13–23. Available from: doi: [PubMed: 17233664] [CrossRef]
- Rand C.S. Non-adherence with asthma therapy: more than just forgetting. The Journal of Pediatrics. [Online] 2005;146(2):157–159. Available from: doi: [PubMed: 15689896] [CrossRef]
- Ratip S., Modell B. Psychological and sociological aspects of the thalassemias. Seminars in Hematology. 1996;33(1):53–65. [PubMed: 8714585]
- Ratip S., Skuse D., Porter J., Wonke B., et al. Psychosocial and clinical burden of thalassaemia intermedia and its implications for prenatal diagnosis. Archives of Disease in Childhood. [Online] 1995;72(5):408–412. Available from: doi: [PMC free article: PMC1511109] [PubMed: 7618906] [CrossRef]
- Riewpaiboon A., Nuchprayoon I., Torcharus K., Indaratna K., et al. Economic burden of beta-thalassemia/Hb E and beta-thalassemia major in Thai children. BMC Research Notes. [Online] 2010;3(1):29. Available from: doi: [PMC free article: PMC2835719] [PubMed: 20181056] [CrossRef]
- Rikos N., Giannadaki G.-K., Spontidaki A., Tzagkaraki M., et al. Health status, anxiety, depression, and quality of life of patients with thalassemia. Journal of Public Health. [Online] 2020 Available from: doi: [Accessed: 15 February 2021] [CrossRef]
- Rofail D., Abetz L., Viala M., Gait C., et al. Satisfaction and adherence in patients with iron overload receiving iron chelation therapy as assessed by a newly developed patient instrument. Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research. [Online] 2009;12(1):109–117. Available from: doi: [PubMed: 18637142] [CrossRef]
- Roy T., Chatterjee S.C. The experiences of adolescents with thalassemia in West Bengal, India. Qualitative Health Research. [Online] 2007;17(1):85–93. Available from: doi: [PubMed: 17170246] [CrossRef]
- Sadowski H., Clemente C., Tsiantis J., Lee C., et al. Psychopathology in children from families with blood disorders: a cross-national study. European Child & Adolescent Psychiatry. [Online] 2002;11(4):151–161. Available from: doi: [PubMed: 12444424] [CrossRef]
- Saini A., Chandra J., Goswami U., Singh V., et al. Case control study of psychosocial morbidity in beta thalassemia major. The Journal of Pediatrics. [Online] 2007;150(5):516–520. Available from: doi: [PubMed: 17452227] [CrossRef]
- Shaligram D., Girimaji S.C., Chaturvedi S.K. Psychological problems and quality of life in children with thalassemia. Indian Journal of Pediatrics. [Online] 2007a;74(8):727–730. Available from: doi: [PubMed: 17785893] [CrossRef]
- Shaligram D., Girimaji S.C., Chaturvedi S.K. Quality of life issues in caregivers of youngsters with thalassemia. Indian Journal of Pediatrics. [Online] 2007b;74(3):275–278. Available from: doi: [PubMed: 17401267] [CrossRef]
- Shega J.W., Andrew M., Hemmerich J., Cagney K.A., et al. The relationship of pain and cognitive impairment with social vulnerability--an analysis of the Canadian Study of Health and Aging. Pain Medicine (Malden, Mass.). [Online] 2012;13(2):190–197. Available from: doi: [PubMed: 22239723] [CrossRef]
- Shi L., Liu J., Fonseca V., Walker P., et al. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health and Quality of Life Outcomes. [Online] 2010;8:99. Available from: doi: [PMC free article: PMC2944346] [PubMed: 20836888] [CrossRef]
- Sobota A., Yamashita R., Xu Y., Trachtenberg F., et al. Quality of Life in Thalassemia: A Comparison of SF-36 Results from the Thalassemia Longitudinal Cohort to Reported Literature and the US Norms. American journal of hematology. [Online] 2011;86(1):92–95. Available from: doi: [PMC free article: PMC4250926] [PubMed: 21061309] [CrossRef]
- Sperber C.M., Samarasinghe S.R., Lomax G.P. An upper and lower bound of the Medication Possession Ratio. Patient preference and adherence. [Online] 2017;11:1469–1478. Available from: doi: [PMC free article: PMC5590759] [PubMed: 28919719] [CrossRef]
- Starke M. Parents needs’ for knowledge concerning the medical diagnosis of their children. J Child Health Care. 2002;61:245–257. [PubMed: 12503895]
- Steinberg-Shemer O., Yacobovich J., Cohen M., Cabantchik I.Z., et al. Labile plasma iron as an indicator of patient adherence to iron chelation treatment. Blood Cells, Molecules & Diseases. [Online] 2018;71:1–4. Available from: doi: [PubMed: 29395830] [CrossRef]
- Taher A., Al Jefri A., Elalfy M.S., Al Zir K., et al. Improved treatment satisfaction and convenience with deferasirox in iron-overloaded patients with beta-Thalassemia: Results from the ESCALATOR Trial. Acta Haematologica. [Online] 2010;123(4):220–225. Available from: doi: [PubMed: 20424435] [CrossRef]
- Thavorncharoensap M., Torcharus K., Nuchprayoon I., Riewpaiboon A., et al. Factors affecting health-related quality of life in Thai children with thalassemia. BMC Blood Disorders. [Online] 2010;10:1. Available from: doi: [PMC free article: PMC2836992] [PubMed: 20180983] [CrossRef]
- Thompson R.H. The handbook of child life: a guide for pediatric psychosocial care. Springfield, Ill.: Charles C. Thomas; 2009.
- Trachtenberg F., Foote D., Martin M., Carson S., et al. Pain as an emergent issue in thalassemia. American journal of hematology. [Online] 2010;85(5):367–370. Available from: doi: [PMC free article: PMC5753761] [PubMed: 20232403] [CrossRef]
- Trachtenberg F., Vichinsky E., Haines D., Pakbaz Z., et al. Iron Chelation Adherence to Deferoxamine and Deferasirox in Thalassemia. American journal of hematology. [Online] 2011;86(5):433–436. Available from: doi: [PMC free article: PMC4599708] [PubMed: 21523808] [CrossRef]
- Trachtenberg F.L., Martin M., Green S., Oliveros O., et al. Use of electronic data collection to assess pain in thalassaemia: a feasibility study. International Journal of Palliative Nursing. [Online] 2012a;18(9):441–445. Available from: doi: [PMC free article: PMC4394650] [PubMed: 23124054] [CrossRef]
- Trachtenberg F.L., Mednick L., Kwiatkowski J.L., Neufeld E.J., et al. Beliefs about chelation among thalassemia patients. Health and Quality of Life Outcomes. [Online] 2012b;10:148. Available from: doi: [PMC free article: PMC3545841] [PubMed: 23216870] [CrossRef]
- Treadwell M.J., Weissman L. Improving adherence with deferoxamine regimens for patients receiving chronic transfusion therapy. Seminars in Hematology. [Online] 2001;38(1) Suppl 1:77–84. Available from: doi: [PubMed: 11206966] [CrossRef]
- Tsiantis J., Dragonas T., Richardson C., Anastasopoulos D., et al. Psychosocial problems and adjustment of children with beta-thalassemia and their families. European Child & Adolescent Psychiatry. [Online] 1996;5(4):193–203. Available from: doi: [PubMed: 8989558] [CrossRef]
- Uman L.S., Chambers C.T., McGrath P.J., Kisely S. A systematic review of randomized controlled trials examining psychological interventions for needle-related procedural pain and distress in children and adolescents: an abbreviated cochrane review. Journal of Pediatric Psychology. [Online] 2008;33(8):842–854. Available from: doi: [PMC free article: PMC2493507] [PubMed: 18387963] [CrossRef]
- Vardaki M.A., Philalithis A.E., Vlachonikolis I. Factors associated with the attitudes and expectations of patients suffering from beta-thalassaemia: a cross-sectional study. Scandinavian Journal of Caring Sciences. [Online] 2004;18(2):177–187. Available from: doi: [PubMed: 15147481] [CrossRef]
- A serial mediating effect of perceived family support on psychological well-being.[BMC Public Health. 2024]A serial mediating effect of perceived family support on psychological well-being.An J, Zhu X, Shi Z, An J. BMC Public Health. 2024 Apr 2; 24(1):940. Epub 2024 Apr 2.
- Social Support and Psychological Distress among Chronic Pain Patients: The Mediating Role of Mindfulness.[Pers Individ Dif. 2022]Social Support and Psychological Distress among Chronic Pain Patients: The Mediating Role of Mindfulness.Wilson JM, Colebaugh CA, Flowers KM, Meints SM, Edwards RR, Schreiber KL. Pers Individ Dif. 2022 May; 190. Epub 2022 Feb 4.
- NEW AND EMERGING PROFESSIONALS: Does Race Moderate Social Support and Psychological Distress Among Rural Older Adults?[Clin Gerontol. 2015]NEW AND EMERGING PROFESSIONALS: Does Race Moderate Social Support and Psychological Distress Among Rural Older Adults?Hyams AV, Wayde EN, Crowther MR, Scogin FR. Clin Gerontol. 2015; 38(5):412-427. Epub 2015 Jul 6.
- Psychological Abuse and Social Support in Chinese Adolescents: The Mediating Effect of Self-Esteem.[Front Psychol. 2022]Psychological Abuse and Social Support in Chinese Adolescents: The Mediating Effect of Self-Esteem.Chen C, Ji S, Jiang J. Front Psychol. 2022; 13:852256. Epub 2022 Mar 24.
- Social support and psychological well-being in younger and older adults: The mediating effects of basic psychological need satisfaction.[Front Psychol. 2022]Social support and psychological well-being in younger and older adults: The mediating effects of basic psychological need satisfaction.Shin H, Park C. Front Psychol. 2022; 13:1051968. Epub 2022 Nov 25.
- Psychological support - 2021 GuidelinesPsychological support - 2021 Guidelines
Your browsing activity is empty.
Activity recording is turned off.
See more...