Copyright © 2024 - Canadian Agency for Drugs and Technologies in Health. Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International licence (CC BY-NC-ND).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
What Is the Issue?
- Gonorrhea is the second most prevalent sexually transmitted infection in Canada. It is caused by Neisseria gonorrhoeae and can be treated with antibiotic therapy. However, N. gonorrhoeae has developed antibiotic resistance, which may decrease the efficacy of current therapy.
- Antibiotic therapy can be administered after a positive N. gonorrhoeae test, but the turnaround time of laboratory testing may result in patients being lost to follow-up (i.e., not returning to the clinic after test results are available).
- Presumptive or empiric antibiotic therapy can be given before the laboratory confirmation of N. gonorrhoeae to individuals at high risk of gonorrhea or those with uncertain follow-up; however, such treatment may lead to overtreating those without N. gonorrhoeae, increasing the risk of antibiotic resistance and possible side effects to the individuals.
- It is important to understand the ideal timing of antibiotic therapy that balances concerns of antibiotic resistance and timely patient care.
What Did We Do?
- To inform decisions about timing of antibiotic therapy for the treatment of adults and adolescents with suspected uncomplicated N. gonorrhoeae infection, CADTH sought to identify and summarize literature comparing the clinical effectiveness and safety of delaying antibiotic therapy until confirmatory results of testing for N. gonorrhoeae infection are available, versus empiric treatment before test results are available.
- A research information specialist conducted a literature search of the peer-reviewed and grey literature published since January 1, 2013.
What Did We Find?
- We did not find any studies directly evaluating the clinical effectiveness and safety of delayed antibiotic treatment compared to presumptive treatment for uncomplicated N. gonorrhoeae infections in adult and adolescent populations. We included 2 nonrandomized studies that compared the rates of accurate treatment and overtreatment in individuals who received presumptive treatment and those who did not.
- In the 2 studies, N. gonorrhoeae test positivity rates in the presumptive treatment group were less than 50%, suggesting that less than half of the patients in this group received accurate presumptive treatment. We also found high overtreatment rates, which were up to 90% in the included studies.
- The certainty of these findings is very low due to methodological limitations of the included studies.
- We also identified 5 single-arm studies that evaluated these outcomes in individuals who received presumptive therapy. The findings are generally consistent with the 2 included studies.
What Does It Mean?
- Available evidence points to high rates of overtreatment when presumptive antibiotics are given. Results also suggest that there is value in clinical assessment in detecting N. gonorrhoeae infections. The downstream clinical effectiveness implications of these results for antimicrobial resistance or increasing spread of N. gonorrhoeae are unclear.
- Contextual factors, such as the local prevalence of N. gonorrhoeae infection and potential barriers to care that could hinder post-test follow-up for certain individuals or groups, may also be useful considerations when making decisions about appropriate timing for N. gonorrhoeae antibiotic therapy.
Context and Policy Issues
What Is Neisseria Gonorrhoeae Infection?
Gonorrhea is a sexually transmitted infection (STI) caused by the bacterium Neisseria gonorrhoeae.1 It is the second most reported bacterial STI in Canada.2 In 2020, there were about 81 new cases per 100,000 individuals living in Canada; 63% of these cases were reported in male patients, and 37% of cases were reported in female patients.3 Uncomplicated gonorrhea infections are urogenital, anogenital, pharyngeal, and ocular gonococcal infections that are not associated with bacteremia or ascending spread of the pathogen to other organs.1 Untreated gonorrhea can lead to serious complications regardless of the presence or severity of symptoms, such as epididymitis and pelvic inflammatory disease.1
Accurate and timely gonorrhea case identification based on symptoms may be challenging because the symptoms may overlap with other disorders such as chlamydia (caused by Chlamydia trachomatis),4 which is the most common STI in Canada.3 For example, both gonorrhea and chlamydia can have genital or extragenital symptoms.4 Symptoms in female individuals (e.g., dysuria and increased vaginal discharge) are often mild and could be mistaken for a bladder or vaginal infection.5 Moreover, some people may have asymptomatic gonorrhea. For example, about 10% of gonococcal urethritis cases in males6 and nearly half of cervicitis cases in females caused by gonorrhea are asymptomatic.7 Asymptomatic rectal and pharyngeal infections are also common in males and females.8
What Is the Current Practice?
Suspected N. gonorrhoeae infection can be confirmed by microscopy of Gram-stained samples, bacterial cultures, or nucleic acid amplification tests (NAATs).1 NAATs are highly sensitive for N. gonorrhoeae, but it may generate false-positive results due to potential cross-reaction with Neisseria meningitidis or other Neisseria species.9
All confirmed gonorrhea cases should be treated with antibiotics.10 However, gonorrhea has developed resistance to previously recommended treatment options including sulfonamides, penicillins, earlier generation cephalosporins, tetracyclines, macrolides, and fluoroquinolones.11 Due to the potential risk of further antimicrobial resistance, the Public Health Agency of Canada currently recommends a combination antibiotic treatment of a third-generation cephalosporin (e.g., ceftriaxone, cefixime) plus either azithromycin or doxycycline, preferably given in a single dose.10 As azithromycin can be given in a single dose (thereby improving treatment adherence) and is effective against C. trachomatis infections, it is preferred over doxycycline.10 The 2020 Centers for Disease Control and Prevention (CDC) treatment guidelines for N. gonorrhoeae infection recommend a single 500 mg intramuscular dose of ceftriaxone. Combination therapy with doxycycline is recommended when C. trachomatis infection cannot be ruled out.12
In Canada, it is recommended to treat all confirmed cases, and to consider treatment in suspected cases, such as individuals at high risk of infection (e.g., partners of known cases, equivocal Gram stain results) or in those individuals with uncertain availability for follow-up.10
What Are Delayed and Presumptive Antibiotic Therapies?
Delayed antibiotic therapy refers to administering antibiotics only after the test results confirm the existence of N. gonorrhoeae. Laboratory testing for N. gonorrhoeae usually has a turnaround time of several days. For example, the turnaround time for NAATs is up to 3 days13 and for N. gonorrhoeae culture is up to 5 days from receipt at the Public Health Ontario laboratory.13 This turnaround time may result in delayed treatment and increase patient loss to follow-up (i.e., not returning to the clinic after test results are available). It could also result in spread to sexual partners in the meantime and pose a risk of developing complications (e.g., pelvic inflammatory disease and infertility in females), if left untreated.14
Presumptive or empirical antibiotic treatment refers to treating suspected N. gonorrhoeae infections with antibiotics before a positive laboratory test. This approach could lead to overuse of antibiotics contributing to antimicrobial resistance, and unnecessary adverse events in individuals who do not actually have an N. gonorrhoeae infection.
Why Is It Important to Do This Review?
Amid growing concerns of antimicrobial resistance globally, there is high interest in antimicrobial stewardship that promotes coordinated interventions to improve the judicious use of antibiotics including selection, dose, and duration of therapy. Considering increasing incidence of N. gonorrhoeae infection Canada,15 a focus on appropriate and effective treatment strategies, as well as a balance between unwanted treatments and timely patient care are important.
This same-day presumptive treatment could help avoid treatment delays, and minimize patient loss to follow-up.14 On the other hand, there are concerns that the presumptive treatment approach may lead to overtreating uninfected individuals, increasing the risk of adverse events such as allergic reactions14 and antibiotic resistance.16,17 It is unclear whether or not delaying antibiotic therapy until confirmatory results of testing are available may have better clinical effectiveness and safety outcomes compared to empiric treatment before test results are available.
Objective
We prepared this Rapid Review to summarize and critically appraise the evidence identified from medical databases and grey literature regarding clinical effectiveness of delayed antibiotic treatment compared to empiric or presumptive treatment for uncomplicated N. gonorrhoeae infection.
Research Question
What is the clinical effectiveness and safety of delaying antibiotic therapy until after confirmatory results of testing for N. gonorrhoeae infection are available versus empiric or presumptive treatment before test results are available in adults and adolescents with suspected uncomplicated N. gonorrhoeae infection?
Methods
Literature Search Methods
An information specialist conducted a literature search on key resources including MEDLINE via Ovid, Embase via Ovid, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO, the Cochrane Database of Systematic Reviews, the International HTA Database, and the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search approach was customized to retrieve a limited set of results, balancing comprehensiveness with relevancy. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. Search concepts were developed based on the elements of the research questions and selection criteria. The main search concepts were gonorrhea (N. gonorrhoeae) and at least 1 synonym for either delayed treatment or presumptive treatment. No filters were applied to limit the retrieval by study type. Conference abstracts were excluded from the search results. Where possible, retrieval was limited to the human population. The search was completed on December 1, 2023, and limited to English-language documents published since January 1, 2013.
Selection Criteria and Methods
One reviewer screened citations and selected studies. Titles and abstracts were reviewed in the first round of screening and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in Table 1, they were duplicate publications, or they were published before 2013.
Critical Appraisal of Individual Studies
The included publications were critically appraised by 1 reviewer using the following tool as a guide: the Downs and Black checklist18 for randomized and nonrandomized studies. Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Summary of Evidence
Quantity of Research Available
A total of 688 citations were identified in the literature search. Following screening of titles and abstracts, 661 were excluded, and 27 potentially relevant reports from the electronic search were retrieved for full-text review. One potentially relevant publication was retrieved from the grey literature search. Of the potentially relevant publications, 26 publications were excluded for various reasons. This report includes 2 nonrandomized studies.19,20 Study selection details are presented in Appendix 1.
We also identified 5 noncomparative single-arm studies.21-25 While these studies did not meet study design eligibility criteria for this report and were not formally included, they provided content relevant to the research question. The characteristics and findings of the single-arm studies are summarized in Appendix 5.
Appendix 6 presents additional references of potential interest related to N. gonorrhoeae and C. trachomatis screening and treatment. These additional studies include a previous CADTH report, single-arm studies of mixed populations with N. gonorrhoeae and C. trachomatis infection, review articles, and studies investigating perspectives of providers and patients.
Summary of Study Characteristics
We included 2 nonrandomized studies in this report.19,20 While we did not identify any studies that reported between-group statistical comparisons of delayed antibiotic treatment versus presumptive antibiotic therapy, the 2 included studies19,20 provided some relevant information about individuals who received presumptive treatment and those who did not.
One of the studies included patients tested for C. trachomatis or N. gonorrhoeae infections.19 Relevant to the current report, we have summarized the characteristics and findings related to the N. gonorrhoeae infections in the following sections. Additional details regarding the included publications are provided in Appendix 2.
Study Design
The study by Shover et al. (2018)20 was a cross-sectional study of medical records. Wilson et al. (2017)19 conducted a prospective cohort study as a part of a noninferiority trial comparing 2 diagnostic tests for C. trachomatis and N. gonorrhoeae infections.
Study Settings and Country of Origin
Both studies were conducted in the US.19,20
Data for the study by Shover et al. (2018)20 was collected from a community-based organization that provides services, including STI testing and treatment, to members of 2SLGBTQ+ communities. The study by Wilson et al. (2018)19 was conducted in an emergency department of an urban academic medical facility. The study periods for the studies were from 201519,20 to 2016.19
Patient Population
Shover et al. (2018)20 enrolled cisgender adult men who have sex with men (MSM) [wording from original source] who were tested for gonorrhea of the urethra, rectum, and/or pharynx. The units of analysis were patient clinic visits (hereafter reported as patients or participants in this report). Altogether, 9,141 visits (for 6,756 unique patients) were included in the study. They ranged from asymptomatic individuals with exposure to gonorrhea to those presenting with clinical signs and symptoms of N. gonorrhoeae infection. Most of the visits were by patients aged 30 years or older who identified as gay or homosexual [wording from original source]. The most commonly reported clinical feature was genitourinary symptoms (11% of visits), and about 11% were asymptomatic contacts.
Wilson et al. (2017)19 included all patients (at any age) who tested for suspected C. trachomatis or N. gonorrhoeae infection. A total of 1,162 patients were included in the study, of which 96% (n = 1,112) were female and 4% (n = 50) were male. Among them, 30% were pregnant (n = 338). The mean age of patients was 26 years (standard deviation [SD] not reported). For each patient, presence of symptoms and signs such as dysuria or urethral or vaginal discharge, cervicitis, or uterine tenderness on exam were assessed; however, no data were reported. About 4% of the patients were asymptomatic.
Interventions and Comparators
Both studies reported outcomes in patients who received presumptive antibiotic treatment and those who did not.19,20
Presumptive Antibiotic Therapy
In the study by Shover et al. (2018),20 empiric or presumptive antibiotic treatment was given according to CDC’s gonorrhea treatment guidelines of 2015 (dual therapy of azithromycin and ceftriaxone). They were given to patients with clinical signs and symptoms of C. trachomatis or N. gonorrhoeae infection and to those with known exposure. Patients with pharyngitis, who reported condomless oral sex with a partner of unknown STI status were also given the presumptive antibiotic treatment. The details of presumptive antibiotic therapy were not reported in the second study.19
No Presumptive Antibiotics
The comparator group in both studies consisted of patients who did not receive presumptive antibiotic treatments.19,20 Neither study described the number of patients who received delayed treatment within that group.
In the study by Shover et al.,20 the comparator group included patients who tested positive and received subsequent delayed treatment, as well as those who tested negative. The proportion of patients who received the delayed treatment was not reported.
Patients in the study by Wilson et al.19 were not followed beyond the emergency department encounter to determine if those who tested positive for N. gonorrhoeae in this group had subsequent follow-up or delayed treatment.
Testing Method
Both studies used NAATs to detect possible N. gonorrhoeae infection.19,20 While urethral, rectal, and pharyngeal swabs were collected in 1 study,20 cervical or urethral swabs were used in the other.19
Outcomes
Outcomes assessed in the 2 nonrandomized studies included:
Summary of Critical Appraisal
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
The study objectives and methods were clearly described in both studies.19,20 The outcomes of interest were described along with their definitions, which were appropriate. Patients in both intervention and control groups were recruited from the same population and over the same period of time. All eligible visits during the study periods were included in the studies, without any sampling.
The included studies had several important limitations that could lower the internal and external validity of the results.19,20 Because both studies were observational studies (retrospective chart review20 and prospective observational19), no randomization of patients was performed. Patients were grouped based on the intervention received, as per the clinical assessment by the health care providers. Thus, treatment and comparator groups were not similar at baseline, making between-group comparison of outcomes challenging. There was no blinding of the patients or the researchers measuring the outcomes. However, because the intervention and outcome measures were objective, the effect of this limitation on the results could be minimal. Another major limitation of the studies was in how the comparator group was defined. In both studies, patients who were not given the presumptive antibiotic treatment were grouped into the control group. However, it was unclear how many patients within that group went on to receive delayed treatment, were lost to follow-up and treatment, or tested negative. In the study by Shover et al.,20 the unit of analysis in the study was “visits,” rather than individual patients. If an individual had multiple visits, they would be considered as separate patient visits and thus counted multiple times. There were 9,141 visits by 6,756 individuals included in the study. It was unclear if individuals who attended the clinic multiple times for the same episode of infection were counted separately. The outcomes relevant to the current report were descriptive, except for the N. gonorrhoeae test positivity rate outcome. Effect estimates or confidence intervals were not reported in the comparative results relevant to the current report. Regional prevalence of N. gonorrhoeae was not accounted for in either study while interpreting the findings.19,20
Accuracy of presumptive treatment was an outcome of interest in both studies.19,20 The studies defined it as the proportion of patients who received presumptive treatment and tested positive for N. gonorrhoeae. The “accuracy” was estimated based on NAAT results of samples collected from the patients. The sensitivity and specificity of NAATs were not factored in while measuring the accuracy of treatment. Because NAATs may have false-positive results,26 it is possible that the true accurate treatment rates could be lower than what was measured in the studies.
As for the external validity of the results, in the Wilson et al. study,19 it was unclear whether patients were representative of the recruited population. The study was conducted among the patients of a larger study that required urine samples. Patients who did not provide a urine specimen were excluded from the larger trial and therefore not included in the Wilson et al. study.19 It is unclear how many were excluded for this reason, and whether the excluded patients were systematically different from the study participants.19 Additionally, 96% of the study population were female,19 whereas in Canada, more than half of newly diagnosed N. gonorrhoeae cases are in males.2
In both studies, it was unclear whether the staff, locations, and facilities where the patients were treated were representative of the treatment most patients receive.19,20 One was conducted in a sexual health clinic serving primarily gay men, bisexual men, and other MSM,20 and the other in an urban academic emergency department.19 The former study primarily included members of the 2SLGBTQ+ community27 and the setting of the latter study may not reflect clinical practice in Canada, where STI testing is mostly conducted in sexual health clinics, local public health units, and walk-in clinics, or by primary health care providers.28 It was reported in the study by Shover et al.20 that patients were presumptively treated based on CDC treatment guidelines at the time (2015), which were comparable to current Canadian recommendations.10 Details of the treatment given were not described in the study by Wilson et al.;19 therefore, we were unable to determine if it was generalizable to current Canadian clinical settings.
Summary of Findings
Appendix 4 presents the main study findings.
Clinical Effectiveness of Delayed Antibiotic Treatment
N. Gonorrhoeae Test Positivity Rates
Shover et al.20 reported that the N. gonorrhoeae test positivity rates in the presumptive treatment and no presumptive treatment groups were 31% and 9%, respectively. The probability of testing positive was statistically significantly higher in patients who received presumptive treatment (P < 0.01). However, presumptive treatment was given based on clinical assessment of signs and symptoms (in addition to the medical and sexual history), which were more prevalent in the presumptive group. For example, compared to 11% in the control group, 52% of visits in the presumptive treatment group included patients reporting genitourinary symptoms.
In the study by Wilson et al. (2017),19 4% of total patients (n = 52 out of 1,162) were N. gonorrhoeae–positive. Among female patients, the test positivity rates were 8% and 1% in the presumptive treatment and no presumptive treatment groups, respectively. While 9% of male patients and 7% of pregnant patients who received presumptive treatment were N. gonorrhoeae–positive, no male or pregnant patients who did not receive same-day treatment had positive test results.
Accurate Presumptive Treatment
Accurate presumptive treatment was defined in the studies as the proportion of patients who tested positive for N. gonorrhoeae among all the patients who received presumptive treatment.
Across the included studies,19,20 the accurate presumptive treatment rate varied. It was 31% in the study by Shover et al.,20 while in the Wilson et al. study,19 it was 8% for male patients and 9% for female patients. Among patients who were pregnant, 7% who received presumptive treatment were N. gonorrhoeae–positive.20
Overtreatment
Overtreatment was defined in the studies as the proportion of patients who received presumptive treatment but tested negative in the laboratory test.
Results from the study by Wilson et al.19 showed that most of the patients who received presumptive treatment tested negative for N. gonorrhoeae, and were thus overtreated. The unnecessary treatment rates were 91% in males, 92% in females, and 93% in individuals who were pregnant.
Missed Treatment
Missed treatment was defined in 1 included study19 as the population who did not receive presumptive treatment but tested positive. It constituted the subset of patients in the “no presumptive treatment” group who tested positive.
In the Wilson et al. study,19 missed treatment rates were low. Out of 640 female patients who did not receive presumptive treatment, 1% (n = 9) tested positive for N. gonorrhoeae and therefore missed treatment, as per the study definition. Since the patients were not followed up, it is unknown whether these patients received subsequent treatment based on their lab results. There were no male or pregnant patients in the “no presumptive treatment” group who tested positive.
Limitations
In the absence of studies that specifically compared delayed antibiotic treatment to presumptive antibiotic treatment, we included studies that compared presumptive treatment with no presumptive treatment. While the “no presumptive treatment” group could include the subset of patients who received “delayed treatment,” the studies included in this review did not provide information about this subset. No randomized studies were identified. None of the included studies evaluated key clinical effectiveness outcomes such as time to treatment, clinical cure, or quality of life, and focused instead on outcomes that related to N. gonorrhoeae test result proportions in various subsets of the study populations. No safety outcomes were reported. We did not identify specific evidence regarding the effectiveness of delayed or presumptive treatment in the adolescent population. Apart from the methodological limitations of the included studies, as described in the Summary of Critical Appraisal section, the external validity of results was low. Both studies were conducted in the 2015 to 2016 period, and 1 study reported that their presumptive treatment was based on the CDC guidelines from 2015. The guidelines have since changed in the US. Moreover, since none of the studies were conducted in Canada, the generalizability to current Canadian clinical settings is unclear.
Conclusions and Implications for Decision- or Policy-Making
Summary of Evidence
This report aimed to summarize the evidence regarding the clinical effectiveness and safety of delayed antibiotic treatment compared to presumptive or empiric antibiotic treatment among adults and adolescents with suspected uncomplicated N. gonorrhoeae infection. We included 2 nonrandomized studies19,20 that provided rates of N. gonorrhoeae test positivity, accurate presumptive treatment, and overtreatments or undertreatments among groups of patients that either received presumptive treatment or no presumptive treatment. We also identified 5 single-arm studies21-25 that reported similar outcomes in presumptively treated patients and have summarized them separately in Appendix 5.
We did not identify comparative evidence (randomized or nonrandomized) regarding relevant clinical effectiveness outcomes such as clinical cure, time to treatment, medication adherence, and quality of life, or safety outcomes between presumptive treatment and delayed antibiotic treatment. We did not identify specific evidence regarding the effectiveness of delayed or presumptive treatment in the adolescent population.
The test positivity rates were numerically higher in the presumptive treatment group than in the no presumptive treatment group,19,20 suggesting that the clinical assessment of signs and symptoms, as well as an assessment of medical and sexual history, is helpful in identifying possible N. gonorrhoeae infections. The positive predictive value of clinical assessment was estimated as high as 56% for genitourinary signs (Table 5).20 However, it should be noted that the positive predictive value of a diagnostic test or clinical assessment is linked to population prevalence of N. gonorrhoeae in the population. In the 2 included studies,19,20 the proportion of patients who were given presumptive treatment and tested positive for N. gonorrhoeae infection (i.e., accurate treatment rates) was reported as less than 10%19 or 31%,20 suggesting that less than half individuals who received presumptive treatment for NG actually required it. In the single-arm studies, the accurate treatment rates similarly ranged from 0%23 to 46.1%.25 One included study reported overtreatment rates of 90%.19 The rates of overtreatment were higher across the single-arm studies as well (68%25 to 100%23). While interpreting these results, it should be noted that factors such as the regional prevalence of N. gonorrhoeae and the diagnostic accuracy of NAATs were not considered in the studies. It is possible that some results were false positives or false negatives, lowering the validity of the results. Furthermore, it was unclear from some studies whether the patients had other STIs with similar clinical presentation (such as C. trachomatis) warranting presumptive treatment, as per the guidelines at the time of the studies.
Among the patients who did not receive presumptive antibiotic treatment, the positivity rates were reported as less than 1%19 and 9%.20 While these studies did not further report on outcomes in patients in the no presumptive treatment group, we identified 1 single-arm study22 reporting that, out of 31 individuals who did not receive presumptive treatment but subsequently tested positive for N. gonorrhoeae infection, 41.9% (n = 13) did not receive treatment.19
Implications for Clinical Practice
In individuals with suspected uncomplicated N. gonorrhoeae infection, the limited evidence identified for this report suggests that overtreatment rates are high. The test positivity rates in the presumptively treated group were numerically higher than in those not presumptively treated, highlighting the value of clinical assessment in detecting N. gonorrhoeae infections. However, the implications of these observations on clinical effectiveness downstream remains undetermined. There is a lack of comparative evidence comparing clinical outcomes between delayed antibiotic treatment and presumptive antibiotic treatment. The subsets of those who received delayed treatment within the no presumptive treatment groups were not reported in the studies.19,20 Overall, due to the methodological limitations in the available studies (e.g., no between-group statistical comparisons, descriptive results only, insufficient details about the treatment given), the certainty of the evidence is very low.
In the absence of clear clinical effectiveness evidence, prevalence of N. gonorrhoeae infections, and the facilitators and barriers to accessing timely testing, treatment, and follow-up as needed, are important decision-making factors. It is important to balance the concerns about antimicrobial resistance and possible adverse effects of unwanted treatment in suspected individuals, as well as the repercussions of not providing timely care to those who need it. Development of accurate point-of-care diagnostic tests could alleviate some of these concerns by eliminating the time between testing and treatment.29
Considerations for Future Research
Well-designed cohort studies with adequate follow-up periods could investigate potential harms associated with delayed treatment — such as missed treatment, loss to follow-up, and spread to contacts — and those of presumptive treatment, such as adverse events and antibiotic resistance. Research focused on equity-deserving groups who may face barriers in accessing follow-up treatments is important.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- NAAT
nucleic acid amplification test
- STI
sexually transmitted infection
Contributors: Elizabeth Carson
References
- 1.
- Seña AC C, MS. Treatment of uncomplicated gonorrhea (Neisseria gonorrhoeae infection) in adults and adolescents. Waltham (MA): UpToDate; 2023: https://www
.uptodate .com/contents/treatment-of-uncomplicated-gonorrhea-neisseria-gonorrhoeae-infection-in-adults-and-adolescents. Accessed 2023 Nov 29. - 2.
- Public Health Agency of Canada. Report on sexually transmitted infection surveillance in Canada, 2019. 2022; https://www
.canada.ca /en/public-health/services /publications /diseases-conditions /report-sexually-transmitted-infection-surveillance-canada-2019.html#s5. Accessed 2024 Jan 12. - 3.
- Public Health Agency of Canada. Table 1. Reported rates of chlamydia, gonorrhea and infectious syphilis per 100,000 population overall and by sex in Canada, 2011 to 2020. In: Chlamydia, gonorrhea and infectious syphilis in Canada: 2020. 2023; https://www
.canada.ca /en/public-health/services /publications /diseases-conditions /chlamydia-gonorrhea-infectious-syphilis-canada-2020-infographic.html#t1ch3. Accessed 2024 Jan 12. - 4.
- Van Ommen CE, Malleson S, Grennan T. A practical approach to the diagnosis and management of chlamydia and gonorrhea. CMAJ. 2023;195(24):E844-e849. [PMC free article: PMC10281205] [PubMed: 37336564]
- 5.
- U.S. Centers for Disease Control and Prevention. Gonorrhea – CDC Detailed Fact Sheet. 2023; https://www
.cdc.gov/std /gonorrhea/stdfact-gonorrhea-detailed.htm#ref8. Accessed 2024 Jan 12. - 6.
- Ong JJ, Fethers K, Howden BP, et al. Asymptomatic and symptomatic urethral gonorrhoea in men who have sex with men attending a sexual health service. Clin Microbiol Infect. 2017;23(8):555-559. [PubMed: 28257898]
- 7.
- Barberá MJ, Serra-Pladevall J. Gonococcal infection: an unresolved problem. Enfermedades Infecciosas y Microbiología Clínica (English Edition). 2019;37(7):458-466. [PubMed: 30732970]
- 8.
- Public Health Agency of Canada. Gonorrhea guide: risk factors and clinical manifestations. 2022; https://www
.canada.ca /en/public-health/services /infectious-diseases /sexual-health-sexually-transmitted-infections /canadian-guidelines /gonorrhea /risk-factors-clinical-manifestation .html. Accessed 2024 Jan 12. - 9.
- Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49(10):3610-3615. [PMC free article: PMC3187337] [PubMed: 21813721]
- 10.
- Public Health Agency of Canada. Gonorrhea guide: treatment and follow-up. 2022; https://www
.canada.ca /en/public-health/services /infectious-diseases /sexual-health-sexually-transmitted-infections /canadian-guidelines /gonorrhea /treatment-follow-up.html#a1. Accessed 2024 Jan 12. - 11.
- Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27(3):587-613. [PMC free article: PMC4135894] [PubMed: 24982323]
- 12.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1911-1916. https://www
.ncbi.nlm .nih.gov/pubmed/33332296. Accessed 2023 Dec 18. [PMC free article: PMC7745960] [PubMed: 33332296] - 13.
- Public Health Ontario. Chlamydia trachomatis/Neisseria gonorrhoeae (CT/NG) – Nucleic Acid Amplification Testing (NAAT). 2023; https://www
.publichealthontario .ca/en/Laboratory-Services /Test-Information-Index /Chlamydia-trachomatis-NAAT-Swabs. Accessed 2024 Jan 12. - 14.
- Nsuami MJ, Lillis RA, Martin DH. Reconsidering presumptive Neisseria gonorrhoeae treatment for women with cervicitis. Sex Transm Dis. 2020;47(6):383-386. [PubMed: 32149957]
- 15.
- Public Health Agency of Canada. Chlamydia, gonorrhea and infectious syphilis in Canada: 2021 surveillance data update. 2023; https://www
.canada.ca /en/public-health/services /publications /diseases-conditions /chlamydia-gonorrhea-infectious-syphilis-2021-surveillance-data.html. Accessed 2024 Jan 18. - 16.
- Fingerhuth SM, Bonhoeffer S, Low N, Althaus CL. Antibiotic-Resistant Neisseria gonorrhoeae spread faster with more treatment, not more sexual partners. PLoS Pathog. 2016;12(5):e1005611. [PMC free article: PMC4872991] [PubMed: 27196299]
- 17.
- Antibiotic resistance threats in the United States. Atlanta (GA): U.S. Centers for Disease Control and Prevention; 2013: https://www
.cdc.gov/drugresistance /pdf/ar-threats-2013-508 .pdf. Accessed 2024 Jan 12. - 18.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [PMC free article: PMC1756728] [PubMed: 9764259]
- 19.
- Wilson SP, Vohra T, Knych M, et al. Gonorrhea and chlamydia in the emergency department: continued need for more focused treatment for men, women and pregnant women. Am J Emerg Med. 2017;35(5):701-703. [PubMed: 28073612]
- 20.
- Shover CL, Beymer MR, Unger EM, Javanbakht M, Bolan RK. Accuracy of presumptive gonorrhea treatment for gay, bisexual, and other men who have sex with men: Results from a large sexual health clinic in Los Angeles, California. LGBT Health. 2018;5(2):139-144. [PMC free article: PMC5833247] [PubMed: 29493405]
- 21.
- Andric B, Drowos J, Trepka MJ, Suciu G, Alonso A, Hennekens CH. High frequencies of negative pretreatment results following presumptive antibiotic treatment for chlamydia and gonorrhea. South Med J. 2013;106(5):321-326. [PubMed: 23644641]
- 22.
- Schechter-Perkins EM, Jenkins D, White LF, Mitchell PM. Treatment of cases of Neisseria gonorrhoeae and Chlamydia trachomatis in emergency department patients. Sex Transm Dis. 2015;42(7):353-357. [PubMed: 26222746]
- 23.
- Friedland SN, Slapcoff B, Dylewski J. Presumptive treatment of chlamydia and gonorrhea infections in a Canadian ambulatory emergency department setting: determination of overtreatment and undertreatment rates. Infect Dis Clin Pract. 2017;25(6).
- 24.
- Pearce E, Chan DJ, Smith DE. Empiric antimicrobial treatment for asymptomatic sexual contacts of sexually transmitted infection in the era of antimicrobial resistance: time to rethink? Int J STD AIDS. 2019;30(2):137-139. [PubMed: 30293504]
- 25.
- Anker B, Jaffar S, Patani H, Bristow CC, Sukhija-Cohen AC. Clinical factors associated with accurate presumptive treatment of Neisseria gonorrhoeae infections in men who have sex with men and transgender women. Clin Infect Dis. 2021;73(9):e3156-e3162. [PMC free article: PMC8563201] [PubMed: 33625487]
- 26.
- Whellams D. Screening for chlamydia and gonorrhea in primary care in populations with low prevalence. CMAJ. 2021;193(33):E1307. [PMC free article: PMC8412425] [PubMed: 34426450]
- 27.
- Everett BG. Sexual orientation disparities in sexually transmitted infections: examining the intersection between sexual identity and sexual behavior. Archives of sexual behavior. 2013;42(2):225-236. [PMC free article: PMC3575167] [PubMed: 22350122]
- 28.
- Public Health Agency of Canada. Getting tested for sexually transmitted infections (STI). 2022; https://www
.canada.ca /en/public-health/services /sexual-health /getting-tested-sexually-transmitted-infections.html#a2. Accessed 2024 Jan 22. - 29.
- World Health Organization. WHO releases new guidance to improve testing and diagnosis of sexually transmitted infections [news release]. 2023 Jul 24.
Appendix 1. Selection of Included Studies
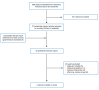
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Table 2
Characteristics of Included Primary Clinical Studies.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Table 3
Strengths and Limitations of Primary Clinical Studies Using the Downs and Black Checklist.
Appendix 4. Main Study Findings
Note that this appendix has not been copy-edited.
Table 4
Summary of Findings by Outcome.
Table 5
Summary of Findings by Outcome — PPV of Signs, Symptoms, and Exposure.
Appendix 5. Summary of Single-Arm Studies
Note that this appendix has not been copy-edited.
Summary of Study Characteristics
We identified 5 noncomparative single-arm studies21-25 describing the clinical effectiveness and potential harms of presumptive antibiotic treatment for gonorrhea. We have summarized characteristics in the subsequent sections. Additional details are provided in Table 6.
Study Design
Of the 5 noncomparative single-arm studies, 4 were retrospective chart reviews,21,22,24,25 and 1 was a retrospective cohort study.23
Study Settings and Country of Origin
One study was conducted in Canada23 and the others were conducted in the US21,22,25 and Australia.24
Study settings included STI testing and HIV or sexual health clinics,21,24,25 as well as emergency departments in urban areas.22,23 The study periods were from 2007 to 2017.21-25
Patient Populations
One study only included individuals tested positive for N. gonorrhoeae or C. trachomatis,22 whereas the other 4 studies investigated those with unconfirmed N. gonorrhoeae infection at baseline.21,23-25 One study collected data from asymptomatic individuals with any STI contact,24 whereas the other 4 studies included individuals with and without symptoms.21-23,25 The number of participants across the 5 studies ranged from 10,024 to 5,051.25 Across studies, most participants were 25 years or older, with 3 studies21,22,24 reporting a mean age ranging from 24.822 to 39 years.24
Among the 3 studies that reported sex,21-23 1 study reported that 99% of participants identified as female,23 and the other 2 studies reported that 55.2%21 and 49%22 of participants identified as male. In the Anker et al. study,25 which included participants identifying as MSM, 99.6% identified as cisgender males and 0.4% identified as transgender (transwomen). One study with data from individuals identifying as MSM did not report sex.24 We have retained the original terms that study authors used when describing sex, gender, and sexual orientation.
Intervention
The intervention of interest in all included single-arm studies was presumptive antibiotic treatment.21-25 Details of presumptive antibiotic treatment therapy for gonorrhea were specified in 4 studies,21,23-25 while the remaining study reported that appropriate antibiotic therapy followed CDC guidelines.22
Summary of Critical Appraisal
The 5 noncomparative single-arm studies clearly reported objectives of the study, patient characteristics, main outcomes to be measured, interventions of interest, and the main findings.21-25 However, none of the studies reported adverse events experienced by individuals who received presumptive treatment but tested negative for N. gonorrhoeae, which is essential to assess potential consequences of overtreatment.21-25
The 5 single-arm studies had methodological limitations due to the lack of a comparator group.21-25 In general, inferring treatment effectiveness from single-arm studies is not possible since there is no alternative to compare it with. None of the studies reported the diagnostic accuracy (e.g., PPV) of NAATs used to confirm N. gonorrhoeae infection.21-25 Potential false-positive and false-negative NAAT test results may increase measurement bias by misclassifying N. gonorrhoeae infection status, introducing a threat to internal validity.
Findings of all 5 studies may have limited external validity.21-25 The representativeness of study populations was unclear. For example, 4 studies recruited participants from a single site,21-24 and 3 of them had a small sample size ranging from 100 to 500,22-24 limiting the representativeness of the patients to the broader population. In addition, the representativeness of study settings was unknown for 2 studies22,23 conducted in emergency departments. While individuals may be screened for STI at emergency departments, STI testing is mostly conducted in sexual health clinics, local public health units, walk-in clinics, or by primary health care providers in Canada.28 Moreover, none of the studies reported regional prevalence of N. gonorrhoeae,21-25 which is critical to assess the generalizability of study findings to populations with various risk levels of N. gonorrhoeae infection.
Summary of Findings
We have summarized findings relevant to the current report in the subsequent sections. Additional details are provided in Table 7.
Accurate Presumptive Treatment
Three studies reported data on accurate presumptive treatment by evaluating the proportion of presumptively treated patients who tested positive for N. gonorrhoeae in various patient populations.23-25 Accurate presumptive treatment ranged from 0%23 to 46.1%,25 which means that less than half of individuals receiving presumptive treatment for N. gonorrhoeae were followed with positive laboratory test results.
One study with patients identifying as MSM and transgender women found:25
- 2,329 of 5,051 (46.1%) individuals received accurate presumptive treatment for N. gonorrhoeae.
One study focusing on asymptomatic MSM participants found:24
- 10 of 38 (26.3%) individuals with N. gonorrhoeae contact alone received accurate presumptive treatment
- 0 of 16 (0%) individuals with N. gonorrhoeae and C. trachomatis contacts received accurate presumptive treatment for N. gonorrhoeae.
One study including male and female participants found:23
- 0 of 19 (0%) individuals receiving presumptive treatment for N. gonorrhoeae tested positive.
Overtreatment
Two studies reported overtreatment between 68%21 to 100%,23 indicating that a large proportion of presumptive treatment for N. gonorrhoeae was unnecessary (i.e., followed with negative laboratory test results).
One of the studies reported a total of 822 of 1,209 (68%) individuals receiving presumptive treatment for N. gonorrhoeae tested negative,21 including:
- 26 of 30 (86.6%) individuals with presumptive treatment for N. gonorrhoeae infection alone
- 402 of 623 (65.2%) individuals with presumptive treatment for both N. gonorrhoeae and C. trachomatis infection.
The other study23 found 19 of 19 (100%) individuals with presumptive treatment for N. gonorrhoeae tested negative. However, the authors acknowledged that overtreatment was high due to the low proportion of positive N. gonorrhoeae results.23
Missed Treatment
One study found that 13 of 31 (41.9%) of individuals without presumptive treatment who tested positive for N. gonorrhoeae did not receive subsequent treatment.22
One study that reported that none of the 19 individuals (0%) without presumptive antibiotics tested positive for N. gonorrhoeae,23 meaning no missed gonorrhea treatment at the initial clinic visit.
Table 6
Characteristics of Relevant Single-Arm Studies.
Table 7
Summary of Findings of Relevant Single-Arm Studies.
Appendix 6. References of Potential Interest
Note that this appendix has not been copy-edited.
- Screening for Chlamydia trachomatis and Neisseria gonorrhoeae during pregnancy: a health technology assessment. Ottawa: CADTH; 2018 Oct. (CADTH health technology assessment report; no. 148) Screening for Chlamydia Trachomatis and Neisseria Gonorrhoeae During Pregnancy: A Health Technology Assessment (cadth.ca) [PubMed: 31145563]
- Allen KS, Hinrichs R, Heumann CL, et al. Findings From a Scoping Review: Presumptive Treatment for Chlamydiatrachomatis and Neisseria gonorrhoeae in the United States, 2006-2021. Sex Transm Dis. 2023 04 01;50(4):209-214. [PMC free article: PMC10006311] [PubMed: 36584164]
- Llata E, Braxton J, Asbel L, et al. Presumptive and Follow-Up Treatment Associated With Gonorrhea and Chlamydia Testing Episodes in Sexually Transmitted Disease Clinics: Impact of Changing Treatment Guidelines for Gonorrhea, Sexually Transmitted Disease Surveillance Network, 2015-2018. Sex Transm Dis. 2023 01 01;50(1):5-10. [PMC free article: PMC10147317] [PubMed: 36194764]
- Rasul R, McIver R, Patel P, Foster R, McNulty A. Non-empirical management of asymptomatic chlamydia and gonorrhoea reduces unnecessary antibiotic use fivefold: a before and after study. Sex Transm Infect. 2023 02;99(1):30-34. [PubMed: 35383124]
- Garlock J, Lee L, Cucci M, Frazee LA, Mullen C. Suspected gonorrhea and chlamydia: Incidence and utilization of empiric antibiotics in a health system emergency department. American Journal of Emergency Medicine. 2019 05;37(5):884-889. [PubMed: 30119987]
- Pugsley RA, Peterman TA. Presumptive and Follow-up Treatment for Gonorrhea and Chlamydia Among Patients Attending Public Health Department Clinics in Virginia, 2016. Sex Transm Dis. 2019 03;46(3):199-205. [PMC free article: PMC10798379] [PubMed: 30742592]
- Breslin K, Tuchman L, Hayes KL, Badolato G, Goyal MK. Sensitivity and Specificity of Empiric Treatment for Sexually Transmitted Infections in a Pediatric Emergency Department. J Pediatr. 2017 10;189:48-53. [PMC free article: PMC5614813] [PubMed: 28629687]
- April MD, Koyfman A, Long B. Symptomatic Emergency Department Patients Should Undergo Empirical Therapy for Gonorrhea/Chlamydia Regardless of Testing. Annals of Emergency Medicine. 2021 04;77(4):410-411. [PubMed: 33766271]
- Unemo M, Ross J, Serwin AB, Gomberg M, Cusini M, Jensen JS. Background review for the '2020 European guideline for the diagnosis and treatment of gonorrhoea in adults'. Int J STD AIDS. 2021 02;32(2):108-126. [PubMed: 33323071]
- Low S, Varma R, McIver R, Vickers T, McNulty A. Provider attitudes to the empiric treatment of asymptomatic contacts of gonorrhoea. Sexual Health. 2020 Apr;17(2):155-159. [PubMed: 32164821]
- McIver R, Low S, Varma R, Vickers T, McNulty A. Empirical treatment of asymptomatic contacts of gonorrhoea: patient views. Sexual Health. 2020 11;17(5):462-466. [PubMed: 33497601]
Previous CADTH Reports
Scoping Review
Nonrandomized Studies
Single-Arm Studies — Mixed Population
Additional References
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up to date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for noncommercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to ac.HTDAC@stseuqeR
- Context and Policy Issues
- Research Question
- Methods
- Summary of Evidence
- Limitations
- Conclusions and Implications for Decision- or Policy-Making
- Abbreviations
- References
- Selection of Included Studies
- Characteristics of Included Publications
- Critical Appraisal of Included Publications
- Main Study Findings
- Summary of Single-Arm Studies
- References of Potential Interest
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Clinical Factors Associated With Accurate Presumptive Treatment of Neisseria gonorrhoeae Infections in Men Who Have Sex with Men and Transgender Women.[Clin Infect Dis. 2021]Clinical Factors Associated With Accurate Presumptive Treatment of Neisseria gonorrhoeae Infections in Men Who Have Sex with Men and Transgender Women.Anker B, Jaffar S, Patani H, Bristow CC, Sukhija-Cohen AC. Clin Infect Dis. 2021 Nov 2; 73(9):e3156-e3162.
- Suicidal Ideation.[StatPearls. 2024]Suicidal Ideation.Harmer B, Lee S, Rizvi A, Saadabadi A. StatPearls. 2024 Jan
- Review Management of gonococcal pelvic inflammatory disease.[Sex Transm Dis. 1979]Review Management of gonococcal pelvic inflammatory disease.Curran JW. Sex Transm Dis. 1979 Apr-Jun; 6(2 Suppl):174-80.
- Review Findings From a Scoping Review: Presumptive Treatment for Chlamydiatrachomatis and Neisseria gonorrhoeae in the United States, 2006-2021.[Sex Transm Dis. 2023]Review Findings From a Scoping Review: Presumptive Treatment for Chlamydiatrachomatis and Neisseria gonorrhoeae in the United States, 2006-2021.Allen KS, Hinrichs R, Heumann CL, Titus MK, Duszynski TJ, Valvi NR, Wiensch A, Tao G, Dixon BE. Sex Transm Dis. 2023 Apr 1; 50(4):209-214. Epub 2022 Dec 30.
- Timing of Antibiotic Therapy for Neisseria Gonorrhoeae InfectionTiming of Antibiotic Therapy for Neisseria Gonorrhoeae Infection
Your browsing activity is empty.
Activity recording is turned off.
See more...
