Copyright © 2023 - Canadian Agency for Drugs and Technologies in Health. Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International licence (CC BY-NC-ND).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
- This review focused on the perspectives and experiences of adults with end-stage renal disease (referred to as “patients”), their families, and their health care providers regarding accessing, offering, deciding about, undergoing, performing, and recovering from procedures to create arteriovenous fistulas (AVFs) for hemodialysis. AVFs are connections between an artery and vein used for vascular access, a process that allows a hemodialysis machine to access a patient’s blood. A total of 8 qualitative studies were synthesized.
- Patients and health care providers mostly valued shared decision-making (SDM) when deciding to undergo procedures to create AVFs. The perceived benefits of SDM include patients’ increased knowledge of their condition, satisfaction, greater sense of control, and improved coping abilities. Yet, some health care providers continue to practice traditional prescriptive approaches to decision-making. Contextual factors influenced decision-making approaches and patients’ agency to access or refuse procedures to create AVFs. These factors included values, beliefs, and attitudes; the timing of decision-making; and human, structural, financial, and informational resources. People who are racialized and those experiencing poverty, houselessness, or language barriers may disproportionately experience difficulties engaging in timely and informed SDM; as a result, they may make uninformed decisions or experience traumatic unplanned dialysis initiation using a form of vascular access they did not choose.
- Decision-makers may consider promoting SDM practices by integrating SDM criteria in health care performance measures and SDM reimbursement models. They may also consider providing decision aids and SDM coaching to health care providers. They may also consider tailored interventions based on unique social, financial, and language-related needs to promote equitable access to procedures to create AVFs.
- During decision-making, patients weigh factors such as trust in their health care providers, past experiences, the invasive nature of procedures to create AVFs, and the anticipated outcomes of these procedures. Patients’ fears of being “cut” or experiencing pain and complications could hinder their engagement in these procedures. Patients’ concerns about an AVF being dysfunctional or hard to maintain and the anticipated pain of needles could also prevent them from wanting AVFs. Additional concerns included the risk of bleeding and an AVF’s impact on physical appearance.
- The included literature provided limited insights into the perspectives and experiences of undergoing, performing, and recovering from procedures to create AVFs. However, some patients and their families experienced financial and emotional burdens while accessing these procedures in Canada. This can be exacerbated by prolonged surgical wait times and rescheduling. People in rural communities, who often had to travel long distances for care, experienced these burdens more than those living in urban areas. Additionally, 1 study reported that surgeons often lead decision-making regarding anesthesia for surgical AVF creation procedures. While considering patient preferences, some health care providers perceive that regional anesthesia made these surgeries easier to perform, potentially resulting in better-quality AVFs. However, barriers to implementing regional anesthesia include limited human resources, funding, and time.
- Finally, patients recovering from procedures to create AVFs reported experiencing pain and fear related to the possibility of never using their AVF. None of the included studies explicitly reported experiences of endovascular procedures to create AVFs. Unlike surgical procedures, these more recent techniques can take place in office-based practices, are noninvasive, and may not cause surgical scarring. Research is needed to explore how implementing endovascular procedures to create AVFs would impact patients’ experiences, outcomes, and access to procedures to create AVFs. Further research is needed to explore health care provider and system barriers to using regional anesthesia.
Context and Policy Issues
Chronic kidney disease (CKD) is characterized by abnormal kidney structure or function that is present for greater than 3 months.1 In the final stage of CKD (i.e., end-stage renal disease [ESRD]), the kidneys no longer function to meet the body’s needs by filtering the waste from blood and excess salt and fluid.1 For this reason, people with ESRD require renal replacement therapy to perform this life-sustaining function.1,2 As of 2021, more than 48, 000 people in Canada were living with ESRD.3
Hemodialysis is a type of renal replacement therapy wherein a person is connected to a machine that filters their blood of excess wastes, salts, and fluids before returning it to them.2,4,5 Vascular access is required for a hemodialysis machine to access a person’s blood, remove it, and return it to them at a high rate.2 Types of vascular access used in hemodialysis include central venous lines (CVLs) (a tube inserted into a large vein), arteriovenous grafts (AVGs) (a synthetic tube joining an artery and vein), and arteriovenous fistulas (AVFs) (a connection created by a vascular surgeon between an artery and vein).4,5 Observational studies have reported that AVFs have longer-term durability and lower association with infections and blood clotting events compared to CVLs and AVGs.6,7 The literature, however, reports a lack of randomized control trial evidence demonstrating the superiority of AVFs and acknowledges that ideal vascular access depends on a patient’s characteristics, values and preferences, life circumstances, and care contexts (e.g., the resources available to support timely access to AVF creation procedures).4,8,9 Recent practice guidelines recommend that clinicians move away from historically encouraged “fistula first” paradigms and emphasize AVFs as the ideal first type of vascular access for people to use for hemodialysis, as well as moving toward creating individualized vascular access plans.10-12 Still, some people with ESRD and their providers may decide that undergoing a procedure to create an AVF is the best option.5
Health care providers create AVFs either through open surgical or minimally invasive endovascular or percutaneous procedures (i.e., endovascular AVF creation [EndoAVF]).13,14 Open surgical procedures involve a vascular surgeon creating an incision in an upper extremity to create an AVF, typically using nonabsorbable sutures.13,14 EndoAVF, a newer approach, involves a health care provider accessing blood vessels through punctures in the skin and guiding an endovascular device to a site where it can be used to create an AVF, either through radiofrequency or thermal energy.13 Whether performed surgically or endovascularly, procedures to create AVFs ideally should take place 3 to 6 months before a patient needs hemodialysis to allow time for any necessary surgical revision and for the site to mature so it can be accessed using a needle for hemodialysis.5,13 However, as detailed by Brown et al.,15 patients may experience barriers to accessing or benefiting from these procedures. Of note, the authors reported that certain equity-deserving groups, such as older adults, females, and people who are Black, living in rural and remote communities, or without health insurance may experience disproportionately lower rates of timely access to AVFs and successful AVF maturation.15
Traditional open surgical AVF creation procedures require operating room time. However, resource constraints may result in extended surgical wait times, limiting the number of patients who can initiate hemodialysis with AVF. When unable to receive timely access to a functional AVF, patients may receive a CVL, which, as detailed earlier, may be associated with higher rates of risks such as infections.6,7 The novel, minimally invasive EndoAVF procedure may reduce costs and wait times associated with surgery. EndoAVF may also be safe, although evidence regarding its clinical efficacy may be limited in quantity and quality.16,17
As decision-makers deliberate on the value of adopting EndoAVF procedures in their jurisdictions, this qualitative rapid review provides a nuanced understanding and synthesis of the perspectives, expectations, and experiences of adults aged 18 years and older with ESRD, their families, and their health care providers regarding accessing, offering, deciding about, undergoing, performing, and recovering from surgical or endovascular procedures to create AVFs for hemodialysis. Additionally, it will explore equity considerations regarding accessing, using, and experiencing benefit from these procedures, as detailed in these perspectives, expectations, and experiences.
Recognizing Indigenous Peoples’ experiences, values, needs, and priorities is crucial for understanding and advancing the state of health technologies and health services in Canada. They also play a vital role in guiding decision-making concerning surgical or endovascular procedures to create AVFs for hemodialysis access. In the interest of fostering culturally safe practices and following careful consideration, we have determined that it would not be appropriate to seek input from Indigenous Peoples regarding their perspectives and experiences for the following reasons. The rapid nature of this review precluded the ability to engage with Indigenous Peoples and Knowledges appropriately. Due to the limited time frame available for establishing respectful and meaningful relationships with Indigenous Peoples to inform this work, CADTH acknowledges that any efforts to incorporate Indigenous Knowledges and voices would not be culturally appropriate or safe. Moreover, such attempts may inadvertently perpetuate harm. CADTH acknowledges that the lack of engagement with and inclusion of Indigenous perspectives and voices constitutes a major limitation and gap in our work. In the spirit of reconciliation, CADTH is in the process of developing reciprocal relationships and authentic engagement with Indigenous partners to develop a strengths-based approach and process to conduct future work that respectfully explores and incorporates Indigenous Knowledges, perspectives, and experiences.
Research Question
- What are the perspectives, expectations, and experiences of adults aged 18 years and older with ESRD, their families, and their health care providers regarding accessing, offering, deciding about, undergoing, performing, and recovering from surgical or endovascular procedures to create AVFs for hemodialysis?
In addition to the primary research question, the reviewer paid particular attention to equity considerations regarding accessing, using, and experiencing benefit from procedures to create AVFs for hemodialysis, as detailed by people with ESRD, their families, and their health care providers.
Methods
Literature Search Methods
An information specialist conducted a literature search on key resources including MEDLINE and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). The search approach was customized to retrieve a limited set of results, balancing comprehensiveness with relevancy. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The search concepts were developed based on the elements of the research questions and selection criteria. The main search concept was arteriovenous fistula. CADTH-developed search filters were applied to limit retrieval to qualitative studies. The search was completed on October 24, 2023, and limited to English-language documents published since January 1, 2014.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first screening level, they reviewed titles and abstracts and retrieved potentially relevant articles. They based their final selection of full-text articles on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
The reviewer excluded articles if they did not meet the selection criteria outlined in Table 1, such as those focusing only on the experiences of people engaging with AVFs after recovering from procedures to create them. Examples of studies excluded for this reason were those focused on experiences of using or having someone else use a needle to access an AVF for hemodialysis or living with and maintaining an AVF after recovery from the initial procedure to create it. Additionally, the reviewer excluded citations published in languages other than English, grey literature, and duplicate publications. Casey et al.18 published a qualitative systematic review describing patients’ perspectives on vascular access initiation and maintenance in hemodialysis in 2014. For this reason, the reviewer also excluded articles published before 2014.
Critical Appraisal of Individual Studies
The reviewer used the optimized version of the Critical Appraisal Skills Programme (CASP) tool to critically appraise the included studies.19 This tool promotes an efficient and systematic appraisal that acknowledges, accepts, and considers the diverse philosophical underpinnings of qualitative inquiry and the varied approaches and methods they inform.19 The reviewer used the optimized CASP tool’s 11 items as prompts for engaged and critical reflection about the trustworthiness and rigour of the included studies. They did not exclude articles based on their quality but instead critically appraised the included studies to provide readers with insight into their limitations and strengths.
Data Analysis
One reviewer analyzed and synthesized the qualitative findings from the included studies informed by the “rapid best-fit framework” synthesis approach.20 The analysis followed a 3-stage approach, which included the development of an initial analytical framework informed by the research questions and familiarization with the included literature, a deductive and inductive extraction and analysis of the data informed by the initial framework, and the iterative refinement of the framework to reflect inductively identified content and relationships among data and themes.
The reviewer developed the initial framework based on 3 sensitizing categories drawn from the research questions, remaining open to refinement in the following analytical stages. The categories aimed to capture the perspectives, expectations, and experiences of people with ESRD, their families, and health care providers regarding:
- accessing, offering, and deciding about surgical or endovascular procedures to create an AVF, initially conceptualized as the period from contemplating offering or undergoing these procedures until the procedure is scheduled
- performing or undergoing the procedure, initially conceptualized as the period from which a surgical or endovascular procedure to create an AVF is scheduled until it is complete
- recovering from the procedure, initially conceptualized as the period that follows completion of the procedure until a health care provider decides and communicates to a person with ESRD and/or their family that either the AVF has matured, an additional unplanned procedure is required to facilitate the AVF’s maturation, or the AVF will never reach maturation.
The reviewer also used considerations detailed in the Scoping and Evaluation phases of Benkhalti et al.’s Equity Checklist for Health Technology Assessment (ECHTA) to inform which concepts to remain sensitive to during the analysis to capture equity considerations.21
- The reviewer conceptualized equity of access as a fair and just opportunity to receive and offer surgical or endovascular procedures to create AVFs. They conceptualized equity of use as patients’ and families’ fair and just opportunities to experience care when deciding, undergoing, and recovering from AVFs that appropriately addresses their physical, psychosocial, informational, cultural, and spiritual needs.
- Finally, the reviewer conceptualized equity of benefit as fair and just opportunities for patients, families, and health care providers to achieve or maintain the best possible physical, psychosocial, cultural, and spiritual health when undergoing, performing, or recovering from (or supporting a person’s recovery from) surgical or endovascular procedures to create AVFs.
The reviewer remained attuned to potential inequities in access, use, and benefit, and whether distribution disparities or institutional biases and care processes may have contributed to these inequities in the context of surgical or endovascular procedures to create AVFs.21 Instead of using dimensions of equity as a coding framework, they used these elements as concepts to prompt sensitivity to data, allowing for the exploration, description, and reporting of how equity considerations relate to the perspectives on, expectations of, and experiences with these procedures.
To begin analysis, the reviewer first familiarized themselves with the studies by reading and rereading them in their entirety while making marginal notes and memos on initial thoughts and insights in a Microsoft Word document. These initial notes and memos included thoughts to promote reflexivity, descriptions prompting familiarization with the content and how it could be mapped onto the preliminary framework, and preliminary equity considerations related to perspectives on, expectations of, and experiences with surgical or endovascular procedures to create AVFs. The reviewer also annotated reflections on methodological considerations as prompted by the optimized CASP tool to facilitate critical appraisal.
The reviewer then used NVivo1423 to begin line-by-line coding of the text or tables under the Findings sections of the included citations into categories of the preliminary framework. After engagement with the literature, the reviewer noted that experiences of accessing procedures to create AVFs did not discreetly fall into the initial categories of offering or deciding about nor performing and undergoing these procedures. The reviewer iteratively refined conceptualizations within the overarching categories of the preliminary framework to better reflect the experiences and perspectives of engaging with procedures to create AVFs reported in the literature. They inductively assigned codes to data falling under the overarching categories based on content and meaning and considered connections between these codes, resulting in additional subcategories further refining the preliminary framework. They mapped inductively identified equity considerations into the categories and emerging subcategories. The reviewer only coded text relevant to perspectives on, expectations of, and experiences with accessing, offering, deciding about, undergoing, performing, and recovering from surgical or endovascular procedures to create AVFs. During the analysis, they remained attuned to connections between the subcategories, and these connections formed the basis of an outline from which they synthesized descriptive themes. Drawing on their growing familiarity with the dataset built through iterative readings, the reviewer returned to their analytical memos and the primary studies to further develop connections within the data and between the subcategories and categories. By doing so, they continued to refine the framework while writing the findings.
Reflexivity
To uphold qualitative best practice, before screening the citations and throughout the analytical process, the reviewer practised reflexivity by creating memos about their prior experiences, assumptions, and knowledge relevant to perspectives, expectations, and experiences of procedures to create AVFs.24 They used these memos to reflect upon what understandings they might bring to their analysis, as well as those that might inform their analysis and challenge assumptions or interpretations not grounded in the data.
Summary of Evidence
Quantity of Research Available
A total of 214 citations were identified in the literature search. Following screening of titles and abstracts, 193 citations were excluded and 21 potentially relevant reports from the electronic search were retrieved for full-text review. Of these potentially relevant articles, 13 publications were excluded for various reasons, and 8 publications met the inclusion criteria and were included in this report. Appendix 1 presents the PRISMA25 flow chart of the study selection.
Summary of Study Characteristics
The characteristics of the included studies are summarized in the following, and additional details are provided in Appendix 2.
Study Designs
The 8 included studies comprised 5 primary qualitative studies26-30 and 3 mixed-methods studies.31-33 The authors of 4 reported methodologies underpinning their qualitative studies or the qualitative component of a mixed-methods study,32 2 of which used qualitative description,26,32 1 grounded theory,28 and 1 interpretive description.29 Griva et al.27 and Woo et al.30 did not report the methodologies underpinning their primary qualitative studies. Armstrong et al.31 similarly did not specify a methodology underpinning the qualitative component of their parallel mixed-methods study, and Rich et al.33 did not explicitly report a design or methodology underpinning their overall mixed-methods study or its qualitative component. Appendix 2 details the methods used for data collection and analysis.
Settings and Participant Characteristics
A total of 3 of the included studies were conducted in Canada26,29,32 and 1 in the UK,31 both of which have universal health care. There were 3 studies conducted in the US28,30,33 and 1 in Singapore27 within the context of health care systems funded through a mix of private and public funding. Appendix 2 provides additional information on the study settings.
The studies included the perspectives of a combined total of 250 participants. Sample sizes for the qualitative studies and qualitative component of the mixed-methods studies ranged from 10 participants33 to 105 participants.27 Participants included:
- 150 people living with ESRD, “advanced,” or stage 4 CKD who were deciding about or engaging with procedures to create vascular access for hemodialysis
- 23 family members or informal caregivers
- 77 health care providers, including,
- 39 physicians specializing in nephrology (n = 16), surgery (n = 10), anesthesiology (n = 7), primary care (n = 3), or interventional radiology (n = 3)
- 31 nurses caring for people with CKD who were deciding about or engaging with procedures to create vascular access for hemodialysis
- 3 “kidney coordinators,” 1 “vascular access coordinator,” 1 social worker, 1 advanced practice provider, and 1 physician assistant.
Except for Armstrong et al.,31 all authors reported the demographic characteristics of the participants. Across studies clearly reporting participants’ age ranges, people with CKD and their families or informal caregivers’ ages ranged from 2628 years to 84 years30 and health care provider’s ages ranged from 37 years to 63 years.28 The authors of only 2 studies29,32 including patients or their families reported the sex or gender of all included participants. Of the combined total of 27 patients and family members included in these studies, 51.9% (n = 14) were reported as female and 48.1% (n = 13) as male. The authors of only 1 study32 reported the sex or gender of all included health care providers (n = 7), all of whom were nurses. Of these providers, 85.7% (n = 6) were reported as female. None of the studies explicitly reported including patients, family members, or health care providers identifying as gender diverse.
Three of the included studies26,28,33 reported insights into their participants’ races or ethnicities, socioeconomic status, or levels of educational attainment. Together, these studies gathered perspectives from diverse participants, including people who were racialized persons; those who were experiencing underemployment, low income, or houselessness; and people who had not completed high school or experienced language barriers when navigating the health care system. None of the included studies explicitly reported including the perspectives of Indigenous people or members of the 2SLGBTQ+ community. Appendix 2 reports details about participant characteristics.
Summary of Critical Appraisal
Of the 8 included studies, all were of moderate to high quality. Three includes a narrative summary and Table 3 details the strengths and limitations of the included studies.
Findings
The included studies did not always separately report the perspectives of people with ESRD from those of people with stage 4 CKD. Therefore, the remainder of this report refers to people with CKD accessing or receiving health care for their condition as “patients.” The findings of this synthesis consist of 3 overarching categories, which include participants’ perspectives, experiences, and understandings regarding:
- offering or deciding about procedures to create AVFs, which includes experiences from contemplating offering or undergoing these procedures until choosing whether to perform or undergo them
- undergoing or performing procedures to create AVFs, which includes experiences from choosing to undergo or perform these procedures until they are complete
- recovering from procedures to create AVFs, which includes experiences in the period that followed the completion of the procedure until a health care provider decided and communicated to a patient and/or their family that either the AVF had matured, an additional unplanned procedure was required to facilitate the AVF’s maturation, or the AVF would never reach maturation.
The studies in this review primarily detailed perspectives and experiences with deciding about these procedures within the broader context of choosing between different types of vascular access. As a result, the findings likewise have a similar focus.
Offering and Deciding About Procedures to Create AVFs
Participants’ perspectives and experiences provided insight into different approaches used during decision-making. The context of decision-making (i.e., the conditions and circumstances that decision-making occurred within) influenced these approaches.
Decisional Approaches
The studies reported experiences with a spectrum of decisional approaches ranging from prescriptive to shared decision-making (SDM).26-30,32,33 Prescriptive approaches involved little or no consideration of the preferences and values of patients and/or their families.8,29 In contrast, SDM involved collaborative information exchanged between a health care provider, a patient, and/or their family. Within this SDM exchange:
- Health care providers shared balanced information about all available options and respective benefits and harms, and, in some cases, their insights or recommendations.26,32 Health care providers and patients expected this information to be individualized (i.e., relevant to the patient’s characteristics, life or living situation, and needs).26,32
In SDM, health care providers shared information grounded in their breadth of exposure to vascular access; however, patients had the ultimate authority to decide.26
The extent to which families were involved in SDM processes varied. Some patients valued considering the values and insights of their family members, while others valued their independence or perceived that their families may be burdened by involvement.27,30 Health care providers generally valued engaging family members, who could motivate patients to make decisions about dialysis and vascular access.27
One study28 reported the experiences of deciding about ESRD Life Plans (i.e., plans detailing all of the anticipated renal replacement therapy methods and vascular access procedures that a patient will receive throughout their life) and described experiences with complex SDM. Participants experienced SDM as complex when it involved:
- processing large volumes of novel clinical information
- advanced planning about long-range interventions
- involved numerous decision-makers, as was the case in a multidisciplinary SDM that included clinicians from various disciplines (e.g., general medicine, nephrology, and surgery) making decisions with a patient.28
The perceived benefits of SDM included patients gaining knowledge of their condition, experiencing satisfaction with and investment in decisional processes and the choice made, and being better prepared for procedures to create AVFs.26,28 By participating in SDM, patients could gain a sense of control over their condition and its treatment, which could facilitate their coping with it.29 However, health care providers perceived that SDM did not necessarily result in people with CKD making informed decisions grounded in sufficient knowledge of the benefits and risks of all possible options.32 These perceptions were informed by their experiences observing patients engaging in behaviours they believed indicated they did not understand relevant risks.32 As detailed in the next section, the context of decision-making influenced whether it was prescriptive, shared, or informed.
The Influence of the Context of Decision-Making on Decisional Approaches
Contextual factors influenced decisional approaches that, in turn, influenced patients’ agency to access or refuse access to procedures to create AVFs.26-30,32,33 These factors tended to influence, and be influenced by, each other.
Values, Beliefs, and Attitudes
Health care providers who believed the best type of vascular access depended on a patient’s characteristics, situation, and preferences tended to value and incorporate SDM into their practice.26,28,29 In contrast, providers upholding the traditional “fistula first” paradigm tended not to offer patients alternatives to procedures to create AVFs.28 Some health care providers also perceived that the policies, laws, and performance measures grounded in “fistula first” paradigms could prevent them from providing patients with treatment alternatives.28 In these contexts, some patients reported feeling pressured into undergoing procedures to create AVFs.28,29 One patient reported that their provider discouraged them from seeking a second opinion by saying they would be willing to withdraw their care if they did so.29
In other cases, providers’ beliefs regarding their patients’ eligibility for procedures to create AVFs informed their approach to decision-making.26,28 Some providers noted they did not offer these procedures to patients they perceived would not benefit from them, including older adults, people with multiple comorbidities, or people who did not obtain a functional AVF after receiving these procedures in the past.26 Health care providers, patients, and their families also understood that procedures to create AVFs were not options for patients who urgently needed dialysis but did not already have vascular access.26 In these cases, patients received a CVL, which did not require time for maturation.26
Health care providers’ beliefs regarding their patient’s preferences and capacity for SDM also influenced decisional approaches.28 Some providers reported believing that their patients either desired to be told what to do or lacked the capacity to engage in SDM, especially when making complex decisions like creating an ESRD Life Plan.28 Yet, some patients, lacking treatment information, felt their providers did not value their preferences or overlooked the impact of vascular access decisions on their lives.26,28
The literature provided insight into the perspectives and experiences of groups who may disproportionately face challenges in engaging in informed SDM due to their health care providers’ beliefs and attitudes. Some providers perceived that using tools to assess decisional conflict could allow them to better appreciate their patients’ perspectives.32 They perceived that doing so could moderate the effect of their own biases for a particular vascular access type.32 However, nurses anticipated that language barriers or cognitive impairment would prevent patients from being able to use and benefit from these tools.32 Additionally, Keller et al.28 reported that some “non-White” [language used by study authors; hereon referred to as racialized] patients in the US felt they were less likely to receive information to inform dialysis decisions.33
Timing
The included studies provided insight into how patients and health care providers timed vascular access discussions after a patient had gained knowledge about their CKD prognosis, accepted the imminent need for hemodialysis, and was ready to discuss vascular access.26,27,30
Patients tended to prioritize deciding whether to undergo hemodialysis over decisions about vascular access.26,30 However, accepting the need for dialysis and, therefore, being ready to discuss and make decisions about it was difficult for some patients and their families.27,30 Barriers to having these discussions included patients not experiencing concerning symptoms or preferring to try alternative interventions such as traditional medicine or prayer.27 Given its association with ESRD, other patients and their family members experienced mortality-related fears that made it challenging for them to accept the imminent need for dialysis, with 1 family member describing dialysis as “tantamount to doomsday.”27,30 Others were concerned dialysis would be expensive (when not insured), lead to lost work or social opportunities, and cause discomfort or pain.27 Patients also feared that their dialysis would cause a burden on their families.27
Health care providers perceived their patients’ avoidance of dialysis discussions as the most common barrier to timely access to procedures to create AVFs.27 They generally valued carefully timing these delicate discussions based on their patients’ level of kidney function and prognosis, although they perceived both as difficult to predict.26-28 However, their perceptions regarding best timing differed. Some were concerned that discussing or making recommendations about dialysis too early would cause patients unnecessary emotional distress or a desire to avoid future care.27 Others, however, perceived that having earlier conversations (i.e., before a patient reached stage 4 CKD) could allow a patient to “come to terms” with the possibility of needing hemodialysis and vascular access to be better able to make decisions in advance of needing it.27 Regardless of when these conversations started, health care providers and patients valued them occurring across multiple time points.26 Participants acknowledged that patients could have difficulty processing the complex and emotionally charged information required to inform decision-making about hemodialysis and vascular access.27,28,33 Multiple conversations would allow patients and their families to ask questions, communicate their preferences, and reevaluate decisions based on evolving preferences and experiences.26,28,32
As previously detailed, patients urgently requiring dialysis before deciding whether to undergo procedures to create AVFs received a CVL.26,29 Romyn et al.29 reported that abruptly and urgently starting hemodialysis with a CVL could be “extremely traumatic” for patients. Health care providers were motivated to re-engage patients in SDM following unexpected dialysis starts; however, they acknowledged the need to delay doing so.26,29 They perceived that patients in these contexts could experience information overload, an inability to concentrate due to illness, and difficulty accepting the reality of their situation.26,29 The timing at which they re-engaged a patient in SDM varied. Some reported reevaluating their patient’s satisfaction with their CVL within weeks of starting dialysis; however, 1 patient reported that their provider did not present the option of undergoing a procedure to create an AVF until they had been on dialysis for a year.26,32
Decision-Making Resources
The availability of human, structural, financial, and informational resources also influenced decisional approaches and the treatment options patients had access to. As described in the following sections, available resources could also influence the timing of decision-making.
Health Care Human Resources
A US-based study reported that health care providers and patients alike perceived a lack of health care human resources to support the complex, multidisciplinary SDM required to create ESRD Life Plans.28 Patients reported feeling rushed in their interactions with their kidney care providers, which led to them not having “enough time to go over things.”28 One nephrologist acknowledged it took time to establish rapport with their patients, which they considered important for supporting SDM.28 However, providers perceived themselves as overextended, pressured to see many patients, and lacking this time.28 They also understood that establishing rapport to facilitate SDM took longer in the context of cultural or language challenges.28 Additionally, they were concerned that already overworked staff would need additional training to support complex SDM processes.28
Health care providers also reported that the organization of human health care resources supported or challenged multidisciplinary SDM.28 They noted that already-existing multidisciplinary team meetings and shared electronic health records in large academic settings could facilitate interprofessional information exchange.28 However, they also anticipated that patients may experience less continuity of care across multiple specialists in these settings.28 In contrast, providers working in smaller private practice settings reported having close working relationships with other clinicians because they relied on referrals.28 They anticipated this would facilitate information exchange to support multidisciplinary SDM.28
Financial Incentives and Resources
Clinicians in the US noted the lack of reimbursement policies for the potentially costly multidisciplinary SDM practices necessary for creating ESRD Life Plans.28 They anticipated that this would decrease the appeal of implementing SDM, especially in smaller, private clinics without a large enough patient volume to rationalize substantial investments that result in commensurate returns.28 Some patients perceived financial incentives as impacting their ability to engage in informed SDM.28 Specifically, they believed dialysis centres prioritized and only offered in-centre dialysis over home dialysis (an option that could influence whether they would pursue procedures to create AVFs) to gain higher profits.28
People living with poverty or houselessness also experienced barriers to accessing the predialysis care necessary to consider undergoing procedures to create AVFs in advance.33 As 1 person who attributed their CKD to their substance use disorder described, “I’m still struggling with the things that got me to this point right here. The environment I’m in and the area I stay at, it’s just—it’s bad.”33 Lacking reliable transportation or social support during illness could also prevent patients living with poverty or houselessness from attending their predialysis appointments.33
Informational Resources
The information available in the decision-making environment influenced the extent to which patients and their health care providers engaged in prescriptive, shared, or informed decision-making about procedures to create AVFs and their opportunities to access them.26-30,32,33 While contemplating or reflecting on their decision-making, patients and their families reported using or potentially benefiting from information regarding:
- CKD, different renal replacement therapy options, and the benefits and risks associated with these options
- different types of vascular access and how they function
- details regarding procedures to create different types of vascular access, including whether these procedures require surgery
However, patients and their families reported that their health care providers did not always exchange this information.26-29 At times, clinicians reported a lack of evidence to determine optimal vascular access based on a patient’s characteristics.28 Without available evidence, they perceived to be limited in the type and extent of information they could share or what they could recommend.28 Alongside information verbally presented by their providers, patients also considered that collected through pamphlets, handouts, and hand-drawn images; the internet; and past personal experiences, or the experiences of other patients, family members, or acquaintances.29,30,33 However, some perceived that the internet lacked centralized, trustworthy resources on vascular access.30 Health care providers and patients also reported that misinformation derived from family, friends, and acquaintances could adversely impact patients’ capacities to make informed decisions.27,30,33
Some patients and their families had either undergone kidney replacement therapy counselling, received care from clinicians claiming to tailor information to their patients’ needs, or had previous experience living with other chronic conditions or working in health care.26,27,30 Even these people, however, sometimes had limited knowledge or understanding of the information necessary to engage in informed SDM about undergoing procedures to create AVFs.26,27,30 Patients and their families reported that the clinical language used in the context of decision-making about dialysis was novel to them.30 For this reason, some recommended that providers and decision tools use plain language whenever possible.28 Furthermore, while most participants with limited English proficiency in a study conducted in a safety-net hospital in the US reported easily accessing interpreter services when needed, 1 found it challenging to communicate with their health care providers.33 Of note, people with limited English proficiency in this study attributed their CKD progression, delayed ESRD diagnoses or care, and/or delayed access to procedures to create AVFs to their lack of insurance coverage, inconsistent primary care, limited access to medications, and perceived deficiency in medical knowledge.33 However, Rich et al.33 did not report whether these persons perceived these phenomena as related to language barriers or multiple and potentially intersecting barriers to predialysis care.
Considerations Deliberated During Decision-Making
To determine whether undergoing a procedure to create an AVF would allow them to achieve their treatment preferences and goals, patients considered:
- the trustworthiness of their health care providers and the nature of past experiences with vascular access
- the nature and anticipated short-term outcomes of procedures to create AVFs
Trust and Past Experiences
People with CKD were better able to overcome fears of dialysis and were more likely to choose to undergo procedures to create AVFs if they trusted a provider that recommended doing so.27,29,30 They tended to trust providers they considered a part of “their team” (i.e., those they were familiar and regularly interacted with) or those trusted providers had referred them to.29,30 Providers could foster trust by spending time with and carefully listening and providing thorough information to their patients; having familiarity and experience with creating or managing AVFs; and having trainees and staff that patients perceived as competent.29,30 Trust could also be broken or mistrust reinforced when patients experienced different providers giving contradictory advice or when they perceived clinicians as financially motivated.27,29 Some providers also perceived their patients as more trusting of advice given by physicians rather than other kidney care providers, such as care coordinators.27
Patients also tended to heavily consider information collected through past personal experiences with vascular access or experiences they witnessed or learned about others having.27,29,32,33 In some cases, people trusted information derived from these experiences more than that from their health care providers.32,33 People with positive personal experiences with a vascular access modality other than an AVF valued maintaining the “status quo” and expressed low interest in procedures to create AVFs.26,32 Learning of others positively experiencing AVFs, however, could sway patients toward wanting to undergo procedures to create them.27 However, those who had experienced complications with their vascular access, including an AVF, considered changing their type of vascular access to prevent further complications.26,29,30,32 Similarly, hearing about or witnessing others’ negative experiences with AVFs could deter people from wanting one.26 Of note, negative experiences with AVFs also could reinforce mistrust in health care providers.27
The Nature and Anticipated Short-Term Outcomes of Procedures to Create AVFs
The included literature did not discuss experiences deciding about EndoAVF. However, health care providers, patients, and their families understood surgical procedures to create AVFs as invasive with the potential to cause pain.27,28,33 Patients often reported fearing being “cut” and the possibility of experiencing pain or surgical complications.27,33 In some cases, these fears could lead them to decide not to undergo procedures to create AVFs.27 Additionally, health care providers and patients deemed AVFs acceptable for long-term vascular access for hemodialysis;26 however, they sometimes decided against these procedures when they anticipated needing hemodialysis for only a short period (e.g., when a patient had been approved for peritoneal dialysis or renal transplant).26 As 1 patient described, “For me, it was never a decision or hard decision which [vascular access type] to choose…at the time I had been approved for transplant…It’s [AVF creation] a much more invasive procedure.”26 One nephrologist also observed that “some older patients don’t even want to be bothered with surgeries,” although they did not explicitly postulate why this may be.28
Anticipated Long-Term Outcomes of Procedures to Create AVFs
Patients and their families also considered the anticipated long-term outcomes of procedures to create AVF when contemplating whether to undergo them.27,30,32,33 Some people with CKD and their families valued the idea of having an AVF as a form of vascular access before needing dialysis to prevent the need for an emergency vascular access.27 Others, however, feared that having a mature AVF would mean they would have to start hemodialysis earlier than necessary.27 This fear could result in patients being hesitant to undergo procedures to create AVFs.27
Patients also contemplated the anticipated success of procedures to create AVFs and the viability of AVFs (i.e., the potential for and ease of maintaining a functional AVF) relative to other vascular access options.27,32 Patients and their families understood that procedures to create AVFs sometimes did not result in a usable AVF and anticipated that AVFs would be more difficult to maintain than CVLs.27,31,32 Some patients specifically reported being concerned that an AVF would become blocked without use and worried about the extra care they perceived they would have to protect the AVF during daily activities or sleep.27,30,32 These concerns could contribute to patients’ hesitancy to undergo procedures to create AVFs.27,31,32 However, some health care providers perceived patients’ viability concerns as being secondary to their fear of dialysis.27 131 They reported that when patients overcame dialysis fears, their viability concerns could be resolved or easily addressed through education.27 131
Patients and their families also believed that, compared to a CVL, using an AVF for hemodialysis would be more difficult, time-consuming, and painful.27 131,32,33 Some developed these perceptions while observing other people with AVFs starting hemodialysis.27 131,32 One person, for example, observed that it took less time for nurses to start their dialysis through their CVL than it did for them to start a patient with an AVF on theirs.32 Similarly, witnessing others screaming while receiving needles to access an AVF could lead people to fear pain and desire to avoid pain.32 In some cases, this resulted in them forgoing procedures to create AVFs in favour of using a CVL, which would not require needles.32
Other concerns relevant to patients’ hesitancy included fears of bleeding following AVF use, infiltrations (i.e., blood leaking outside of the AVF to surrounding tissues), or aneurysms (i.e., bulging in an AVF that could lead to it clotting or bursting), and altered physical appearance.27,30,32 Of note, 1 person described choosing an AVG placed in the leg over an AVF placed in their arm, as they anticipated the AVG would be easier to hide.30 In contrast, some patients in the included studies weighed the risk of infection less than other vascular access concerns. Specifically, some reported being aware that the risk of infection was greater with a CVL than with an AVF and that CVL-related infections could lead to death.32 However, when appraising themselves as capable of mitigating the risk of infection with a CVL (e.g., by following instructions to avoid getting it wet), some chose to forgo undergoing procedures to create AVFs.27,32
Undergoing and Performing Procedures to Create AVFs
The experiences of undergoing and performing procedures to create AVFs reported in the literature were limited. The authors of 1 qualitative study29 in British Columbia reported patients’ experiences preparing for and accessing procedures to create AVFs after deciding to undergo them. Another mixed-methods study31 in the UK explored health care providers’ practices related to anesthesia (i.e., medication used to block sensation or awareness temporarily) for surgical procedures to create AVFs. Neither of these studies reported patients’ experiences during these procedures.
Undergoing
Given that procedures to create AVFs were planned, some patients reported feeling they had enough time to receive information from multiple sources to prepare for them.29 However, even with time to prepare, patients and their families reported needing to take time off work and spend money and time travelling to urban centres to receive AVF appointments and creation procedures.29 Patients living in rural communities in British Columbia especially experienced coordinating travel to these centres as a significant challenge that consumed up to 7 hours of their time.29 Some patients depended on social assistance to accommodate this travel, while others reported paying out of pocket as they earned slightly above the qualifying income cut-offs.29 Patients reported the need to accommodate inflexible scheduling by taking the first available appointment.29 However, they frequently experienced prolonged wait times for AVF surgeries that could be rescheduled to accommodate more urgent cases.29 This rescheduling resulted in patients losing additional money and time.29
Accessing procedures to create AVFs could also be emotionally burdensome for patients and their families.29 One patient, for example, described the stress of seeking social assistance to support travel-related expenses: “I fight like crazy with social assistance every time I need to go for surgery, to the point where I’m bawling my eyes out on the phone.”29 Patients and their families living in rural communities also experienced fear for their safety while travelling long distances, often through dangerous weather conditions, to access the tertiary care centres where procedures to create AVFs would take place.29 Finally, patients were grateful for and relied on emotional and practical support from their families and friends as they navigated procedures to create AVFs.29 At the same time, however, they feared they had burdened those closest to them and sometimes withheld their emotions to avoid increasing this perceived burden.29
Performing
The 3 different types of anesthesia health care providers discussed using for surgical procedures to create AVFs included:
- regional anesthesia [RA] nerve blocks, which involved an anesthetist injecting medication near a network of nerves to prevent a patient from feeling the region a surgeon would operate on
- local anesthesia [LA], which typically involved a surgeon injecting anesthesia directly at the site of the surgery
- general anesthesia [GA], which involved giving medications to temporarily alter a patient’s level of consciousness.31
Health care providers largely regarded surgeons as leading decision-making regarding anesthesia, although surgeons considered the preferences of their patients when doing so.31 In contrast, anesthetists were often only involved in decision-making on the day of surgical procedures to create AVFs, except in cases where a surgeon identified a patient as being “particularly high risk.”31 Although many anesthetists perceived surgeons as having the skills for deciding among LA, RA, and GA, some anticipated benefits to being involved earlier.31 One anesthetist reported that patients could be surprised, disappointed, or discontent when being told shortly before the procedure that the type of anesthesia the anesthetist planned to perform differed from what the surgeon told them they would receive.31
Participants reported practising either “LA/mixed” or “RA dominant,” wherein surgical teams typically used LA or RA, respectively.31 When providers in LA/mixed centres deemed LA would be inappropriate (e.g., when cases were “more complex”), they tended to use GA instead. They would, however, use RA when patients had comorbidities that prevented GA from being a suitable option.31 In RA-dominant centres, providers tended to avoid using GA.31
Some surgeons preferred performing procedures to create AVFs with LA because doing so did not require an anesthetist and was faster.31 Some preferring LA also noted they had positive surgical outcomes (e.g., creating functional AVFs at a rate exceeding nationally reported averages) and therefore were not inclined to change their practice.31 Other health care providers perceived RA as preferable, and noted that it prevented patients from moving their arms and could lead to blood vessel dilation.31 These providers perceived that these outcomes could make surgery easier to perform, potentially leading to better-quality AVFs.31 Anesthetists sometimes advocated for using RA following their engagement with supporting trial evidence.31 This advocacy resulted in RA becoming standard practice within their centre once surgeons positively experienced it.31 Of note, health care providers unanimously supported the idea of a randomized control trial providing evidence to support the uptake of the types of anesthesia most likely to lead to improvements in AVF function.31 Despite its perceived practical and clinical benefits, however, a lack of skilled anesthetists, limited financial resources to compensate for increased use of anesthetists’ or operating room time, and limited time to accommodate RA (e.g., due to the volume of cases scheduled) could challenge or hinder RA use.31
Health care providers also acknowledged that their patients’ characteristics and preferences influenced the type of anesthesia used.31 Anesthetists reported being flexible and planning based on patients’ preferences.31 Providers understood GA as being associated with adverse clinical outcomes (such as mortality) and unsuitable for patients with certain comorbidities.31 However, they reported that some patients, particularly those with high anxiety, prefer or need GA so they would be asleep during procedures for creating AVFs.31 Some, however, also perceived that patients were generally more receptive to being awake during procedures after hearing of other patients’ positive experiences.31 One anesthetist commented that patients who had multiple procedures to create AVFs reported positive experiences with RA because, for example, they appreciated not being able to feel their whole arm rather than just the surgical site during the procedure.31
In cases where they used RA, anesthetists chose a specific type of nerve block based on their skill, the anticipated location of surgery on the arm, and their perception of the benefits and risks of each in the context of their patient’s characteristics.31 Most favoured using “supraclavicular blocks.”31
Recovering From Procedures to Create AVFs
Only 1 included study29 (set in British Columbia) explicitly reported the perspectives and experiences of recovering from procedures to create AVFs. It found that patients feared their AVFs would not mature for hemodialysis as they recovered from these procedures and that “pain consistently occurred with AVFs from creation onwards.”29 The authors of this study also reported that people experienced continuous support from their families as essential during hospitalization for procedures to create AVFs.29 However, they did not detail the specific support families provided during the recovery period.
Limitations
This review has limitations that may reduce the trustworthiness and relevance of some of its findings. The reviewer deemed all included citations to be of moderate to high quality. However, none of the included citations explicitly reported on the perspectives and experiences of EndoAVF. Additionally, the findings of most included studies focused broadly on decision-making regarding vascular access for hemodialysis while providing limited insight into the perspectives and experiences of undergoing, performing, and recovering from procedures to create AVFs. The relative novelty of EndoAVF and recent shifts away from the “fistula first” paradigm and toward individualized vascular access plans may explain these phenomena.10-12,34 However, as a result of the focus of the included studies, this review provides limited insights into the perspectives and experiences that may be relevant to inform implementation decisions about procedures to create AVFs generally and EndoAVF specifically.
Additionally, the included studies reported on the perspectives and experiences of people receiving care in Canada, people who are racialized, people of various educational backgrounds, and people living with low income, poverty, houselessness, or language barriers. However, as none of the studies included patients perspectives and experiences, this review is missing experiences and considerations relevant and important to Indigenous people. Of note, Indigenous people in Canada are more likely to experience ESRD, require dialysis, and encounter difficulties accessing kidney care due to systemic and intersecting factors such as racism, colonization, and discrimination.35,36 Their perspectives and experiences would have added important insights relevant to decisions about procedures to create AVFs in Canada.
Further limitations relate to the methods used to complete this rapid review. A single reviewer screened and selected citations, conducted the analysis, and reported the results, all within a rapid time frame. These methods increased the likelihood of the reviewer missing eligible citations or analytical findings and limited their ability to produce an in-depth analysis.
Conclusions and Implications for Decision- or Policy-Making
This best-fit framework synthesis of 8 studies focused on the perspectives, expectations, and experiences of adults with ESRD, their families, and their health care providers regarding accessing, offering, deciding about, undergoing, performing, and recovering from procedures to create AVFs for hemodialysis.
Approaches to making decisions about offering or undergoing procedures to create AVFs ranged from prescriptive to SDM. Prescriptive decision-making involved little or no consideration of patient values and preferences. These approaches, for example, tended to stem from providers adhering to historical “fistula first” paradigms or assuming their patients did not desire SDM or lacked the capacity to engage in it. In contrast, SDM involved the collaborative exchange of information between health care providers, patients, and/or their families, followed by patients deciding which option they trusted would align with their treatment values and goals. The benefits of SDM included patients having increased knowledge of their condition, satisfaction with the decision, and a sense of control, which could facilitate coping. However, contextual factors influenced the extent to which patients could engage in SDM, which in turn influenced their agency to access or refuse access to procedures to create AVFs. The timing and frequency of discussions about dialysis and procedures to create AVFs also influenced decisional approaches. Limitations in human, structural, financial, and informational resources could also lead to prescriptive decision-making. People who are racialized and those experiencing poverty, houselessness, or language barriers may experience disproportionate barriers to access and engaging in timely and informed SDM about procedures to create AVFs and the benefits resulting from this engagement. This, in turn, may place them at an increased risk of experiencing uninformed decision-making or unplanned dialysis starts with CVLs, the latter of which patients could experience as traumatic.
The findings in this review corroborate the barriers to SDM in hemodialysis vascular access practice and the potential solutions that Murea et al.37 previously detailed and proposed. Decision-makers seeking to support SDM in their jurisdictions may consider:
- reevaluating health care performance measures or reimbursement models grounded in the “fistula first” paradigm
- supporting health care curriculums that train providers to support SDM and effectively use tools to elicit patient preferences
- interventions to build or retain human, structural, and financial resources that allow kidney care providers to establish rapport with their patients to support them with their dialysis concerns and initiate vascular access discussions early and over multiple encounters
- supporting research to better understand AVF treatment preferences and outcomes
- interventions to address inequities that some groups may experience in accessing and benefiting from timely, informed SDM and the agency to access (or refuse access to) procedures to create AVFs. These may include interventions that provide health care providers with support in challenging racist assumptions that may lead to them offering people who are racialized fewer treatment options. They may also include interventions that address social, financial, and language barriers to predialysis care. More research exploring these barriers and their intersections may better support decision-making.
The included studies also reported that patients weigh multiple factors when deciding whether to undergo procedures to create AVFs. Patients were more likely to undergo procedures to create AVFs if a provider they trusted recommended them. However, patients often weighed the nature of past experiences with vascular access more heavily than health care providers’ recommendations. Furthermore, both patients and their health care providers considered surgical procedures to create AVFs to be invasive. Patients’ fears of being cut or experiencing pain or complications from these procedures could hinder their desire to engage in them. Patients also considered the anticipated outcomes of these procedures when deciding whether to undergo them. Concerns about an AVF not maturing or being easily maintained and worries about anticipated needle-related pain could prevent patients from wanting AVFs. Additional concerns hindering patients’ engagement in procedures to create AVFs included those regarding the perceived risk of bleeding, infiltrations, and aneurysms, and the anticipated impact an AVF would have on their physical appearance. The relative risk of infection between AVFs and CVLs was of lesser concern to patients.
These findings corroborate those detailed in the extant literature.15,18,38 Considering these concerns, decision-makers would benefit from considering research investigating whether:
- EndoAVF is associated with higher rates of AVF maturation and durability (which is beyond the scope of this review)
- patients may find EndoAVF more appealing or less distressing (e.g., due to its noninvasive nature and reduced risk of surgical scarring) compared to open surgical procedures.34
However, decision-makers may also consider that Casey et al.18 and Fielding et al.38 reported that patients with AVFs experience accessing them with needles as a necessary but painful process that can cause distressing changes to appearance (e.g., due to scars or bruising) and feelings of vulnerability. Having EndoAVF as an option may not address these concerns and burdens, re-emphasizing the relevance of patients engaging in informed SDM that considers their treatment goals and preferences.
This review also found that patients and their families experience challenges when preparing for and accessing procedures to create AVFs. Some felt they had adequate information to prepare for the procedures; however, they experienced financial and time-related burdens to accessing them, exacerbated by prolonged surgical wait times and rescheduling. While some received social assistance to cope with the financial burdens of accessing care, income cut-offs limited access to this support. Accessing procedures was also emotionally burdensome for patients and their families, including for those experiencing stress when attempting to access social assistance and those living in rural areas who faced safety concerns during travel. More research is needed to understand whether and how implementing EndoAVF, which providers can conduct in office-based practices rather than operating rooms, could improve barriers to accessing procedures to create AVFs, including for those in rural areas.34
Finally, 1 included study31 reported that surgeons often led decision-making regarding anesthesia, with consideration for patient preferences. Some surgeons may prefer using LA for its speed and lack of need for an anesthetist; however, others may prefer RA if they perceive that it will make surgery easier to perform and potentially result in a better-quality fistula. Anesthetists who are inspired by evidence suggesting the clinical benefits of RA could encourage surgeons to adopt it; however, challenges like limited skilled human resources, insufficient funding, and time could prevent the adoption of RA despite its perceived benefits. Patient characteristics and preferences also influenced the type of anesthesia used during procedures to create AVFs. Recovery from these procedures involved fears related to the possibility of an AVF not maturing and pain. Considering these findings, decision-makers would benefit from having research that explores whether and how implementing EndoAVF could impact the resources available to support using RA during surgical AVF procedures (e.g., by freeing up surgical resources). Decision-makers also may benefit from research exploring how these procedures impact the experience of postprocedural fear and pain.
This qualitative review exploring the experiences of people engaging with procedures to create AVFs for hemodialysis provides valuable insights into the considerations, values, and concerns most important to them. It provides a nuanced understanding of barriers to accessing, using, and benefiting from these procedures, including whether disparities in experiencing these barriers relate to historical, social, institutional, and environmental disadvantage or discrimination. These understandings are useful for informing funding and implementation decisions regarding these procedure in Canadian jurisdictions with consideration for these values, concerns, barriers, and possible inequities.
Abbreviations
- AVF
arteriovenous fistula
- AVG
arteriovenous graft
- CASP
Critical Appraisal Skills Programme
- CKD
chronic kidney disease
- CVL
central venous line
- EndoAVF
endovascular AVF creation
- ESRD
end-stage renal disease
- GA
general anesthetic
- LA
local anesthetic
- RA
regional anesthetic
- SDM
shared decision-making
Contributor: Francesca Brundisini
References
- 1.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150. https://kdigo
.org/wp-content /uploads/2017 /02/KDIGO_2012_CKD_GL.pdf. Accessed 2023 Oct 31. - 2.
- Berns JS. Patient education: Hemodialysis (beyond the basics) In: Sshwab JJ, ed. UpToDate. Waltham (MA): UpToDate; 2022: http://www
.uptodate.com. Accessed 2023 Oct 31. - 3.
- Canadian Institute for Health Information. Annual statistics on organ replacement in Canada, 2012 to 2021. 2022; https://www
.cihi.ca/en /annual-statistics-on-organ-replacement-in-canada-2012-to-2021. Accessed 2023 Oct 31. - 4.
- Allon M. Vascular access for hemodialysis patients: New data should guide decision making. Clin J Am Soc Nephrol. 2019;14(6):954-961. [PMC free article: PMC6556719] [PubMed: 30975657]
- 5.
- Arasu R, Jegatheesan D, Sivakumaran Y. Overview of hemodialysis access and assessment. Can Fam Physician. 2022;68(8):577-582. [PMC free article: PMC9374077] [PubMed: 35961720]
- 6.
- Ravani P, Gillespie BW, Quinn RR, et al. Temporal risk profile for infectious and noninfectious complications of hemodialysis access. J Am Soc Nephrol. 2013;24(10):1668-1677. [PMC free article: PMC3785277] [PubMed: 23847278]
- 7.
- Ravani P, Palmer SC, Oliver MJ, et al. Associations between hemodialysis access type and clinical outcomes: A systematic review. J Am Soc Nephrol. 2013;24(3):465-473. [PMC free article: PMC3582202] [PubMed: 23431075]
- 8.
- Woo K, Lok CE. New insights into dialysis vascular access: What is the optimal vascular access type and timing of access creation in CKD and dialysis patients? Clin J Am Soc Nephrol. 2016;11(8):1487-1494. [PMC free article: PMC4974877] [PubMed: 27401524]
- 9.
- Quinn RR, Ravani P. Fistula-first and catheter-last: Fading certainties and growing doubts. Nephrol Dial Transplant. 2014;29(4):727-730. [PubMed: 24327565]
- 10.
- Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75(4 Suppl 2):S1-s164. [PubMed: 32778223]
- 11.
- Lok CE, Rajan DK. KDOQI 2019 vascular access guidelines: What is new. Semin Intervent Radiol. 2022;39(1):3-8. [PMC free article: PMC8856770] [PubMed: 35210726]
- 12.
- MacRae JM, Oliver M, Clark E, et al. Arteriovenous vascular access selection and evaluation. Can J Kidney Health Dis. 2016;3:2054358116669125. [PMC free article: PMC5332074] [PubMed: 28270917]
- 13.
- Tyagi R, Ahmed SS, Navuluri R, Ahmed O. Endovascular arteriovenous fistula creation: A review. Semin Intervent Radiol. 2021;38(5):518-522. [PMC free article: PMC8612838] [PubMed: 34853497]
- 14.
- Marsh A, Genova R, Lopez J. Dialysis fistula. Treasure Island (FL): StatPearls Publishing; 2023: https://www
.ncbi.nlm .nih.gov/books/NBK559085/. Accessed 2023 Oct 31. - 15.
- Brown RS. Barriers to optimal vascular access for hemodialysis. Seminars in Dialysis. 2020;33(6):457-463. [PubMed: 33030298]
- 16.
- National Institute for Health and Care Excellence. Interventional procedure overview of percutaneous endovascular forearm arteriovenous fistula creation for haemodialysis access (Interventional procedures program IP1833). 2021; https://www
.nice.org .uk/guidance/ipg710/documents/overview. Accessed 2023 Oct 31. - 17.
- National Institute for Health and Care Excellence. Percutaneous endovascular forearm arteriovenous fistula creation for haemodialysis access (Interventional procedures guidance IPG710). . 2021. https://www
.nice.org .uk/guidance/ipg710/resources /percutaneous-endovascular-forearm-arteriovenous-fistula-creation-for-haemodialysis-access-pdf-1899876092240581. Accessed 2023 Dec 1. - 18.
- Casey JR, Hanson CS, Winkelmayer WC, et al. Patients' perspectives on hemodialysis vascular access: A systematic review of qualitative studies. Am J Kidney Dis. 2014;64(6):937-953. [PubMed: 25115617]
- 19.
- Long H, French D, Brooks J. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Res Methods Med Health Sci. 2020;1(1):31-42. https://journals
.sagepub .com/doi/full/10 .1177/2632084320947559. Accessed 2023 Nov 10. - 20.
- Shaw L, Nunns M, Briscoe S, Anderson R, Coon JA. A “Rapid Best-Fit” model for framework synthesis: Using research objectives to structure analysis within a rapid review of qualitative evidence. Res Synth Methods. 2021;12(3):368-383. [PubMed: 33006277]
- 21.
- Benkhalti M, Espinoza M, Cookson R, Welch V, Tugwell P, Dagenais P. Development of a checklist to guide equity considerations in health technology assessment. Int J Technol Assess Health Care. 2021;37:e17. [PubMed: 33491618]
- 22.
- Braveman P. A new definition of health equity to guide future efforts and measure progress. 2017; https://www
.healthaffairs .org/content/forefront /new-definition-health-equity-guide-future-efforts-and-measure-progress. Accessed 2023 Oct 06. - 23.
- NVivo qualitative data analysis, version 14 [computer program]. Denver (CO): Lumivero; 2022.
- 24.
- Charmaz K. Constructing grounded theory. 2nd ed. London (UK): Sage Publications Ltd; 2014.
- 25.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [PubMed: 19631507]
- 26.
- Elliott MJ, Ravani P, Quinn RR, et al. Patient and clinician perspectives on shared decision making in vascular access selection: A qualitative study. Am J Kidney Dis. 2023;81(1):48-58.e41. [PubMed: 35870570]
- 27.
- Griva K, Seow PS, Seow TY, et al. Patient-related barriers to timely dialysis access preparation: A qualitative study of the perspectives of patients, family members, and health care providers. Kidney Med. 2020;2(1):29-41. [PMC free article: PMC7525138] [PubMed: 33015610]
- 28.
- Keller MS, Mavilian C, Altom KL, Erickson KF, Drudi LM, Woo K. Barriers to implementing the Kidney Disease Outcomes Quality Initiative End-Stage Kidney Disease Life Plan guideline. J Gen Intern Med. 2023;05:05. [PMC free article: PMC10651571] [PubMed: 37407766]
- 29.
- Romyn A, Rush KL, Hole R. Vascular access transition: Experiences of patients on hemodialysis. Nephrol Nurs J. 2015;42(5):445-454. [PubMed: 26591269]
- 30.
- Woo K, Pieters H. The patient experience of hemodialysis vascular access decision-making. J Vasc Access. 2021;22(6):911-919. [PMC free article: PMC8216296] [PubMed: 33118395]
- 31.
- Armstrong RA, Wilson C, Elliott L, et al. Regional anaesthesia practice for arteriovenous fistula formation surgery. Anaesthesia. 2020;75(5):626-633. [PMC free article: PMC7187449] [PubMed: 32030735]
- 32.
- Murray MA, Thomas A, Wald R, Marticorena R, Donnelly S, Jeffs L. Are you SURE about your vascular access? Exploring factors influencing vascular access decisions with chronic hemodialysis patients and their nurses. Cannt J. 2016;26(2):21-28. [PubMed: 29218970]
- 33.
- Rich NC, Vartanian SM, Sharief S, Freitas DJ, Tuot DS. A mixed-methods investigation of incident hemodialysis access in a safety-net population. BMC Nephrol. 2017;18(1):279. [PMC free article: PMC5581413] [PubMed: 28865432]
- 34.
- Vachharajani TJ, Taliercio JJ, Anvari E. New devices and technologies for hemodialysis vascular access: A review. Am J Kidney Dis. 2021;78(1):116-124. [PubMed: 33965296]
- 35.
- Komenda P, Lavallee B, Ferguson TW, et al. The prevalence of CKD in rural Canadian Indigenous Peoples: Results from the First Nations Community Based Screening to Improve Kidney Health and Prevent Dialysis (FINISHED) screen, triage, and treat program. Am J Kidney Dis. 2016;68(4):582-590. [PubMed: 27257016]
- 36.
- Thomas DA, Huang A, McCarron MCE, et al. A retrospective study of chronic kidney disease burden in Saskatchewan's First Nations people. Can J Kidney Health Dis. 2018;5:2054358118799689. [PMC free article: PMC6144512] [PubMed: 30245841]
- 37.
- Murea M, Grey CR, Lok CE. Shared decision-making in hemodialysis vascular access practice. Kidney Int. 2021;100(4):799-808. [PMC free article: PMC8463450] [PubMed: 34246655]
- 38.
- Fielding C, Bramley L, Stalker C, Brand S, Toft S, Buchanan H. Patients' experiences of cannulation of arteriovenous access for haemodialysis: A qualitative systematic review. J Vasc Access. 2023;24(5):1121-1133. [PMC free article: PMC10631276] [PubMed: 35034481]
- 39.
- Lincoln Y, Guba E. But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation. New Dir Eval. 1986;1986(30):73-84. https:
//onlinelibrary .wiley.com/doi/abs/10.1002/ev.1427. Accessed 2023 Nov 11.
Appendix 1. Selection of Included Studies
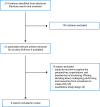
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Table 2
Characteristics of Included Publications.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Narrative Summary of the Findings of the Critical Appraisal
None of the authors of the included studies clearly stated the philosophical assumptions underpinning their study. The authors of 2 studies,26,32 however, reported using a pragmatic methodology agnostic to particular worldviews (i.e., qualitative description). The authors of 4 studies27,30,31,33 also did not report a methodology underpinning their qualitative study or the qualitative component of their mixed-methods study. Keller et al.28 reported using “qualitative methods with grounded theory methodological orientation” but do not specify the type of grounded theory used. Most citations in their methods section relate to constructivist grounded theory; however, the authors also cite Strauss and Corbin’s grounded theory and inductive thematic analysis papers.28 The authors of 3 studies26,28,29 reported using methods that do not typically align with their cited overall methodological approach and/or analytical approach. Congruence between philosophical underpinnings, methodologies, and methods enhances the credibility of a study’s findings, indicating that researchers have the knowledge and skills necessary to conduct qualitative inquiry.39
Chosen qualitative methodologies, when reported, were appropriate for addressing the stated objectives of all 9 studies.
All authors explicitly reported methods used to recruit and select their participants and used a purposeful sampling approach appropriate for gaining access to relevant experiential experts. Except in 1 study,31 all authors reported the characteristics of participants they interviewed. This enhanced the theoretical transferability of the study’s findings by providing information allowing readers to compare settings and participants to their own context.
Except in 2 studies,32,33 all authors explicitly detailed methods of data collection. The authors of 3 studies31-33 did not report sufficient detail to show the methods used for data analysis were rigorous and/or aligned with the cited approach.
All authors provided a statement of ethical approval. The authors of only 2 studies27,30 explicitly reported engaging in reflexive practices and the nature of the relationship between researchers and participants. The authors of all studies explicitly reported methods to enhance the credibility of their findings.
Table 3
Critical Appraisal Using the Optimized CASP Tool.
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for noncommercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to Requests@CADTH.ca.
- Perspectives and Experiences Regarding the Creation of Arteriovenous Fistulas fo...Perspectives and Experiences Regarding the Creation of Arteriovenous Fistulas for Hemodialysis Access: A Rapid Qualitative Review
Your browsing activity is empty.
Activity recording is turned off.
See more...
