Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
- The clinical effectiveness of oseltamivir for the prevention of influenza in residents of long-term care facilities compared to no prophylaxis is unclear. The evidence we identified is inconclusive due to limitations in the quality of studies.
- We did not find any evidence on the safety of oseltamivir for preventing influenza in long-term care facilities.
- We did not find any studies on the clinical effectiveness or safety of oseltamivir prophylaxis in long-term care facilities compared to placebo.
Context and Policy Issues
Influenza is a common acute viral respiratory infection caused by influenza viruses of the Orthomyxoviridae family.1 Worldwide, influenza affects around a billion people every year, resulting in millions of hospitalizations.2 In Canada, it is estimated that influenza results in approximately 3,500 deaths and more than 12,000 hospitalizations annually, making influenza a major public health concern2 and 1 of the top 10 leading causes of death.3 The clinical spectrum of the symptoms and signs of influenza is wide, ranging from asymptomatic infections to severe cases resulting in respiratory, cardiac, gastrointestinal, and other complications, and death.1 Residents of long-term care facilities, nursing homes, or other chronic care facilities are considered a high-risk group for influenza complications and death2 because of their increased age, comorbidities, and shared living conditions.
While influenza is self-resolved and would not require medical care in most affected individuals, people in high-risk groups (e.g., residents of long-term care facilities, individuals with chronic health conditions, adults 65 years of age or older) may require medical care, and treatment.2 Antiviral drugs such as neuraminidase inhibitors reduce the spread of the virus in the respiratory tract by blocking the release of progeny virions.1 Oseltamivir, zanamivir, and peramivir are the neuraminidase inhibitors available for treatment and prophylaxis in Canada.2 In addition to the seasonal influenza vaccines, pre- and post-exposure prophylaxis using antiviral drugs play an important role in controlling the spread of influenza outbreaks in the community, as well as high-risk settings like long-term care facilities.2,4 While prophylaxis with oseltamivir has been found to be protective against symptomatic influenza in community and household settings, the evidence in institutional settings such as long-term care facilities is sparse and unclear.4,5
The purpose of this report Is to summarize the evidence regarding clinical effectiveness of oseltamivir for the prevention of influenza in residents of long-term care facilities.
Research Question
What is the clinical effectiveness of oseltamivir for the prevention of influenza in residents of long-term care facilities?
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the International HTA Database, and the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were oseltamivir, long-term care, and flu prevention. No filters were applied to limit the retrieval by study type. Where possible, retrieval was limited to the human population. The search was completed on October 13, 2022, and limited to English-language documents published since January 1, 2016.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in Table 1, they were duplicate publications, or were published before 2016. Systematic reviews in which all relevant studies were captured in other more recent or more comprehensive systematic reviews were excluded.6
Critical Appraisal of Individual Studies
The included publications were critically appraised by 1 reviewer using the following tools as a guide: A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2)7 for systematic reviews, and the Downs and Black checklist8 for randomized and non-randomized studies. Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Summary of Evidence
Quantity of Research Available
A total of 371 citations were identified in the literature search. Following screening of titles and abstracts, 341 citations were excluded and 30 potentially relevant reports from the electronic search were retrieved for full-text review. No potentially relevant publications were retrieved from the grey literature search for full-text review. Of the potentially relevant articles, 26 publications were excluded for various reasons, while 4 publications met the inclusion criteria and were included in this report. These comprised 1 overview of reviews,9 1 systematic review,10 and 2 non-randomized studies.11,12 Overview of reviews or “umbrella review” refers to evidence synthesis of existing systematic reviews.13 Appendix 1 presents the PRISMA14 flow chart of the study selection.
Additional references of potential interest are provided in Appendix 5.
Summary of Study Characteristics
One overview of reviews,9 1 systematic review,10 and 2 non-randomized studies11,12 were included in this report. Characteristics of included studies are summarized in the following sections.
Both the overview of reviews9 and the systematic review10 had broader inclusion criteria than the present report. Specifically, they evaluated the clinical effectiveness of oseltamivir as treatment or prophylaxis for influenza in a general population (all individuals and settings). Residents of semi-closed or institutional settings (e.g., long-term care homes) were considered as a subgroup of interest in both publications.9,10 None of the included studies in the systematic review were conducted in the population and setting relevant to the current report.10 The overview of reviews identified 1 systematic review which evaluated the effectiveness of oseltamivir prophylaxis in long-term care home residents.9 Only the characteristics and results of the subset of relevant studies will be described in this report.
Additional details regarding the characteristics of included publications are provided in Appendix 2.
Study Design
The overview of reviews by Doll et al.(2017)9 searched for systematic reviews and meta-analyses published from 1995 to 2015 across multiple electronic databases. They included 27 systematic reviews; among which 1 (Rainwater-Lovett et al. [2014]) was relevant to the current report.
The systematic review by Boikos et al. (2017)10 searched for randomized controlled trials and observational studies published from 2009 to 2015. Although 165 studies were included in the systematic review, none were conducted among residents of long-term care facilities.
The included primary studies were a prospective cohort study by Dronavalli et al. (2020)11 and a retrospective cohort study by Shah et al. (2019).12 Both studies were analyses of routinely collected administrative data from influenza outbreaks in long-term care facilities. The study period in the Dronavalli et al. study11 was from 2015 to 2018, whereas the study period in the Shah et al.12 study was the 2017 to 2018 influenza season.
Country of Origin
The authors of the overview of reviews9 and the systematic review10 were from Canada. The non-randomized studies were conducted in Australia11 and England.12
Patient Population
All individuals (general population) were of interest in the overview of reviews.9 Residents of closed or semi-closed institutional settings, including residents of long-term care homes, were considered as a specific subgroup of priority. The number and characteristics of participants within the relevant systematic review (Rainwater-Lovett et al. [2014]) were not reported.9
The participant population in the non-randomized studies comprised residents of care homes12 or aged care facilities11 that had confirmed influenza outbreaks. In the Dronavalli et al.11 study, 10,064 residents from 86 aged care facilities were included in the study. Among them, 4,395 (43.6%) received oseltamivir prophylaxis. In the study by Shah et al.,12 3,498 residents from 109 care homes were included in the study. Among them, 2,200 residents received oseltamivir prophylaxis. The demographic and clinical characteristics of the residents (e.g., age, sex, comorbid conditions such as malignancy or diabetes) were not reported in either study.11,12
Interventions and Comparators
The intervention of interest in all included publications was oseltamivir prophylaxis.9,11,12
The dose and frequency of the intervention was not reported in the overview of reviews.9 In the non-randomized study by Shah et al.,12 oseltamivir prophylaxis was given within 36 to 48 hours of exposure for a duration of 10 days (based on the recommendation by Public Health England). The rate of adherence to these recommendations was unclear.12 The timing, dose, frequency, and duration of oseltamivir prophylaxis given to the participants was not reported in the study by Dronavalli et al.11
In all included publications, oseltamivir prophylaxis was compared to no prophylaxis.9,11,12
Outcomes
The clinical effectiveness outcomes considered in the included publications were secondary transmission (1 overview of reviews),9 attack rate (2 non-randomized studies),11,12 oseltamivir prophylaxis failure (2 non-randomized studies),11,12 rate of hospitalization (1 non-randomized study),12 and mortality (1 non-randomized study).12 A definition for attack rate was not provided in the non-randomized studies;11,12 however, in epidemiological studies, attack rate has been defined as the proportion of individuals in a defined population who get infected during an outbreak.15 Dronavalli et al.11 defined prophylaxis failure as the ratio of attack rate in residents who received oseltamivir prophylaxis to those who did not receive oseltamivir prophylaxis.
Although several safety outcomes were examined in the overview of reviews,9 results specific to the population of interest in the current report (residents of long-term care facilities) were not reported. Safety outcomes were not reported in the non-randomized studies.11,12
Summary of Critical Appraisal
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
Overview of Reviews and Systematic Review
The objectives and inclusion criteria for the overview of reviews9 and the systematic review10 were clearly described. Population, intervention, comparators, and outcomes of interest were clear, and appropriate to the objective of the reviews. The methods of both publications were established a priori in the form of published protocol.9,10 There were some post hoc deviations from the protocol, which were justified. A comprehensive literature search was conducted to identify eligible studies. Multiple electronic databases and reference lists were searched. The search was performed within 24 months of completion of the publications.9,10 Study selection, data extraction, and quality assessment of the included primary studies10 (or individual systematic reviews, in the case of the Doll et al. overview9) was conducted by 2 independent reviewers. Discrepancies were resolved by discussion and a third reviewer. In the overview of reviews,9 the quality of the included systematic reviews was assessed using appropriate and validated tools, such as the AMSTAR questionnaire and the enhanced Overview Quality Assessment Questionnaire (eOQAQ). The Grading of Recommendations Assessment Development and Evaluation (GRADE) framework was used to assess the quality of evidence. In the systematic review,10 risk of bias in the primary studies was assessed using GRADE criteria. Domains such as randomization, allocation concealment, blinding, selection bias, and residual confounding were considered while evaluating the quality of the studies. Neither publication conducted a quantitative synthesis of evidence considering the possible heterogeneity across the individual studies.9,10 Results of the qualitative synthesis were reported clearly in both publications. Limitations of the review were discussed, and conclusions were reported with appropriate caveats.9,10 The overview of reviews by Doll et al.9 had some additional strengths specific to the methodology of overview. The authors used a clear definition of systematic reviews. A reproducible literature search, selection flow chart, and quality assessment of primary studies was considered in defining systematic reviews. Overlap of primary studies across the included systematic reviews was examined and presented using an overlap matrix table. Individual systematic reviews with complete overlap of primary studies with others were excluded. There were no informal indirect comparisons of results across different systematic reviews.9
The overview9 had some limitations as well. Although the authors examined the overlap of primary studies across the systematic reviews, and those with complete overlap were excluded from the overview, there was still up to 35% overlap across primary studies. Because of this, it is possible that aggregate results of narrative synthesis were drawn from data counted two or more times. The detailed characteristics of each included systematic reviews were not reported. Results or conclusions from all included systematic reviews were also not provided. Lastly, the quality of the primary studies across the included systematic reviews was unclear. Methodological limitations of the randomized controlled trials and observational studies would affect the overall validity of the results of the overview.9 The main limitation of the systematic review by Boikos and colleagues10 was that the detailed characteristics and results of included primary studies were not reported. However, considering that they included 165 primary studies, the omission could be due to publication constraints. It was also unclear whether publication biases and their sources (if any) were explored. Sources of funding for individual studies were also not reported.10 Overall, the included overview of reviews and systematic review were of moderate to high quality, and well designed with detailed reporting.9,10
Non-Randomized Studies
The 2 non-randomized studies11,12 were epidemiological outbreak control cohort studies using secondary administrative data from long-term care facilities in Australia11 and England12. The study objectives were clear, and the outcomes of interest were appropriate and clearly defined. It is likely that the staff, places, and facilities where the residents lived were representative of the care that the majority of patients receive.11,12
The studies had several limitations. Since the studies were conducted using administrative data, all residents of care homes that had an influenza outbreak were included in the analysis. There was no randomized allocation, allocation concealment, or blinding of participants. The baseline demographic or clinical characteristics of study participants were not reported. It was unknown whether potential confounding factors such as age, comorbidities (e.g., malignancy, diabetes), or vaccination status were balanced between participants in the intervention and comparator groups.11,12 This could introduce confounding bias. The frequency, dosage, and timing of oseltamivir prophylaxis given to the participants was not reported in 1 study.11 The timing and duration of prophylaxis was reported in the other study.12 However, the individual compliance to the medication was unknown.11,12 Adverse events related to oseltamivir prophylaxis were not reported in either study.11,12 It was unclear whether data on adverse events were captured in the respective administrative databases. There were several reporting issues in the study by Dronavalli et al.,11 such as inconsistent reporting of numerical data and possible percentage calculation errors. For example, the authors reported that the rate of prescribing oseltamivir prophylaxis was 54%; however, it was also reported elsewhere that 4,392 residents out of 10,064 total residents received oseltamivir prophylaxis, which is 43.6%.11 These inconsistencies in reporting lowered the internal validity of the findings. Since the intervention group (oseltamivir prophylaxis group) was defined based on exposure to prophylaxis (oseltamivir), there was a risk of immortal time bias, which was not corrected in the study with a time-dependent variable. Immortal time bias occurs when, by design, participants in the exposed group are considered immortal before the exposure to intervention, since they must survive to receive the intervention and be included in the intervention group.16 As a result of the incorrect management of immortal time, the benefit of oseltamivir prophylaxis may be overestimated in all of the comparisons. Overall, the non-randomized studies were of low quality due to several major limitations.11,12
Summary of Findings
Appendix 4 presents the main study findings.
Clinical Effectiveness of Oseltamivir Prophylaxis Versus No Prophylaxis
Secondary Transmission
The overview of reviews by Doll et al.9 reported results from a systematic review (Rainwater-Lovett et al. [2014]) regarding the effectiveness of oseltamivir prophylaxis in long-term care facilities for the outcome secondary transmission. There were no significant differences in secondary transmission of influenza between residents who received oseltamivir prophylaxis and the residents who did not receive prophylaxis. The results were similar (no significant difference) for different influenza types (influenza A alone, as well as influenza A or B). The analysis was not stratified based on pre-exposure or post-exposure prophylaxis.
There were 10 primary studies in the systematic review by Rainwater-Lovett et al.(2014); however, the number of participants across those studies was unclear.9 Doll et al. reported that the quality of evidence from this systematic review was lowered due to some concerns regarding risk of bias and imprecision.9
Attack Rate
Two non-randomized studies11,12 reported results regarding attack rate, which is the proportion of individuals in a defined population who get infected during an outbreak.
Dronavalli et al.11 found that the attack rate in aged care facilities that used oseltamivir prophylaxis was 17% lower than in facilities where no antiviral prophylaxis was used (1.9% versus 18.9%). The number needed to treat to prevent 1 influenza case was 6. However, results of a between-groups statistical comparison were not reported; therefore, it is unclear whether this difference in rates is statistically significant. Results from the non-randomized study by Shah et al.12 (N = 3,498) showed that the attack rate in the oseltamivir prophylaxis group was significantly higher than in the no prophylaxis group (27% versus 20.1%; P < 0.001). In both studies, instances of influenza-like illnesses were counted when calculating the attack rate. It is unclear how many cases were laboratory-confirmed in the Shah et al. study.12 The inconsistency in these findings could be due to multiple reasons, including: methodological limitations such as confounding bias, patient compliance to prophylactic medication being unclear, limited quality of the data source, or poor definition of influenza cases resulting in possible overestimation.
Oseltamivir Prophylaxis Failure
Oseltamivir prophylaxis failure, defined as the ratio of attack rates in residents who received oseltamivir prophylaxis to those who did not, was calculated and reported by Dronavalli et al.11 The risk ratio of oseltamivir prophylaxis failure was 0.10 (95% CI, 0.08 to 0.12), suggesting that the use of the antiviral drug was associated with a 90% prevention of influenza cases.
When calculated at the facility level (instead of the patient level), it was found that the risk of oseltamivir prophylaxis failure was statistically significantly higher in aged care facilities with a higher prophylactic use.11 The authors proposed that this could be because of confounding by indication. Confounding by indication occurs when there is a bias in the intervention–outcome relationship due to the indication for intervention.17,18 For example, oseltamivir prophylaxis use in the facilities was dictated by outbreak; a more severe outbreak could mean a higher uptake of prophylaxis. A severe outbreak could also result in spread of infection to more individuals, which would affect the outcome attack rate. Validity of facility-level results are further reduced by the likely differences in resident population such as age and comorbidities, and factors such as size, infrastructure, staff, and quality ranking of the long-term care facilities. Results of an analysis adjusting for these factors were not reported.11
Rate of Hospitalization
Rate of hospitalization was reported in 1 non-randomized study of 3,498 participants.12 There was no difference in hospitalization rates due to influenza-related illness between patients who received oseltamivir prophylaxis and patients who did not.
Mortality
Death due to influenza-like illness was reported in 1 non-randomized study of 3,498 participants.12 There was no difference in death rates due to influenza-related illness between patients who received oseltamivir prophylaxis and patients who did not.
Harms
No relevant evidence was identified regarding the harms of oseltamivir prophylaxis compared to no prophylaxis among residents of long-term care facilities; therefore, no summary can be provided.
In their overview of reviews, Doll et al.9 found that treatment or prophylaxis with neuraminidase inhibitors (e.g., oseltamivir, zanamivir) was not associated with a higher risk of adverse events or serious adverse events in the general population. There was a higher risk of side effects such as nausea and vomiting, and a lower risk of diarrhea, associated with oseltamivir use. The authors were inconclusive about the association between neuraminidase inhibitors and neuropsychiatric side effects. However, it should be noted that these conclusions were for a general population in all settings. Harms outcomes specifically in long-term care home settings were not reported.
Clinical Effectiveness of Oseltamivir Prophylaxis Versus Placebo
No relevant evidence was identified regarding the clinical effectiveness of oseltamivir prophylaxis compared to placebo among residents of long-term care facilities; therefore, no summary can be provided.
Limitations
The findings of this report should be interpreted in light of the limitations. Relevant evidence from the overview of reviews9 was limited to 1 systematic review. No relevant studies were included in the systematic review by Boikos et al.10 Although these publications were of moderate to high quality, very little relevant evidence was included in them.9,10 The 2 non-randomized studies11,12 had several major methodological limitations, as described in the Summary of Critical Appraisal section. Therefore, a lack of good-quality evidence regarding the clinical effectiveness of oseltamivir for preventing influenza among residents of long-term care facilities was the main limitation of this report.
No evidence regarding oseltamivir prophylaxis compared to placebo in the setting of long-term care facilities was found. No relevant evidence regarding the harms or safety of oseltamivir prophylaxis compared to no prophylaxis or placebo among residents of long-term care facilities was identified. It was unclear whether the evidence from the overview of reviews9 was based on studies conducted in Canada. The 2 non-randomized studies were conducted in Australia11 and England12; therefore, the generalizability of the findings to Canadian settings is not clear.
Conclusions and Implications for Decision- or Policy-Making
One overview of reviews,9 1 systematic review,10 and 2 non-randomized studies11,12 regarding the clinical effectiveness of oseltamivir prophylaxis in residents of long-term care facilities were included in this report. The overview of reviews9 and the systematic review10 were of moderate to high quality, while the 2 non-randomized studies11,12 had several major methodological limitations. Since the systematic review10 did not include any relevant primary studies, evidence from the overview of reviews9 and the non-randomized studies11,12 were summarized in this report. A systematic review (included in the overview of reviews9) found no differences in the secondary transmission of influenza between long-term care home residents who received oseltamivir prophylaxis and residents who did not receive oseltamivir prophylaxis. Evidence from 1 non-randomized study11 suggested that oseltamivir prophylaxis was associated with a prevention of 90% of influenza cases compared to no prophylaxis. However, due to probable confounding bias, immortal time bias, and methodological limitations, the validity of the results is very low. The evidence regarding the effectiveness of oseltamivir prophylaxis on attack rate (proportion of individuals in a defined population who get infected during an outbreak) of influenza was inconsistent and very uncertain.11,12 A non-randomized study found no differences in hospitalization rates and mortality due to influenza-like illness between residents who received oseltamivir prophylaxis and those who did not receive oseltamivir prophylaxis.12 No evidence regarding harms of oseltamivir prophylaxis compared to no prophylaxis among residents of long-term care facilities was identified. No evidence regarding the clinical effectiveness and harms of oseltamivir prophylaxis compared to placebo among residents of long-term care facilities was identified.
Influenza is a major public health concern associated with significant clinical1 and economic implications.19 This report highlights the lack of high-quality studies appropriately designed to examine the effectiveness of oseltamivir prophylaxis in long-term care home settings. A CADTH report published in 2017 concluded that oseltamivir was effective in preventing influenza in a general population.20 Considering the risk of neuropsychiatric21 and other serious adverse events associated with oseltamivir, the decision to use oseltamivir in high-risk populations should be based on a risk–benefit analysis. Well-designed future studies are warranted in this population to assess the clinical effectiveness and safety of oseltamivir prophylaxis.
References
- 1.
- Uyeki TM, Hui DS, Zambon M, Wentworth DE, Monto AS. Influenza. Lancet (London, England). 2022;400(10353):693-706. [PMC free article: PMC9411419] [PubMed: 36030813]
- 2.
- Government of Canada. Flu (influenza): For health professionals 2022; https://www
.canada.ca /en/public-health/services /diseases/flu-influenza /health-professionals.html. Accessed 2022 Nov 7. - 3.
- Canada S. Deaths, 2019. 2020; https://www150
.statcan .gc.ca/n1/daily-quotidien /201126/dq201126b-eng.htm. Accessed 2022 Nov 8. - 4.
- Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza and other Respiratory Viruses. 2017;11(5):356-366. [PMC free article: PMC5596516] [PubMed: 28691237]
- 5.
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2019;68(6):e1-e47. [PMC free article: PMC6653685] [PubMed: 30566567]
- 6.
- Yuan Y, Wang RT, Xia J, Cao HJ. Interventions for preventing influenza: An overview of Cochrane systematic reviews and a Bayesian network meta-analysis. J. 2021;19(6):503-514. [PubMed: 34544670]
- 7.
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [PMC free article: PMC5833365] [PubMed: 28935701]
- 8.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [PMC free article: PMC1756728] [PubMed: 9764259]
- 9.
- Doll MK, Winters N, Boikos C, Kraicer-Melamed H, Gore G, Quach C. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: a systematic review of systematic reviews and/or meta-analyses. Journal of Antimicrobial Chemotherapy. 2017;72(11):2990-3007. [PubMed: 28961794]
- 10.
- Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. Journal of Antimicrobial Chemotherapy. 2017;72(6):1556-1573. [PubMed: 28204554]
- 11.
- Dronavalli M, Lord H, Alexander K, Boonwaat L, Pal N, Fletcher-Lartey SM. Effectiveness of Oseltamivir Prophylaxis in Influenza Outbreaks in Residential Aged Care. J Epidemiol Glob Health. 2020;10(2):184-189. [PMC free article: PMC7310780] [PubMed: 32538036]
- 12.
- Shah A, Harries AD, Cleary P, McGivern M, Ghebrehewet S. Use of prophylactic antivirals and care home characteristics associated with influenza in care homes with confirmed outbreaks. Public Health. 2019;177:48-56. [PubMed: 31533085]
- 13.
- Pollock M FR, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, ed. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022): Cochrane; 2022.
- 14.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [PubMed: 19631507]
- 15.
- Centers for Disease Control and Prevention. Principles of epidemiology in public health practice: centers for disease control and prevention. 2012; https://www
.cdc.gov/csels /dsepd/ss1978/glossary.html. Accessed 2022 Nov 7. - 16.
- Catalogue of Bias Collaboration. Immortal time bias. 2020; https:
//catalogofbias .org/biases/immortal-time-bias/. Accessed 2022 Nov 7. - 17.
- Joseph KS, Mehrabadi A, Lisonkova S. Confounding by Indication and Related Concepts. Current Epidemiology Reports. 2014;1(1):1-8.
- 18.
- Catalogue of Bias Collaboration. Confounding by indication. 2018; https:
//catalogofbias .org/biases/confounding-by-indication. Accessed 2022 Nov 7. - 19.
- de Courville C, Cadarette SM, Wissinger E, Alvarez FP. The economic burden of influenza among adults aged 18 to 64: A systematic literature review. Influenza and Other Respiratory Viruses. 2022;16(3):376-385. [PMC free article: PMC8983919] [PubMed: 35122389]
- 20.
- The use of antivirals for influenza prophylaxis: a review of the clinical effectiveness. Ottawa (ON): CADTH; 2017: https://www
.cadth.ca /use-antivirals-influenza-prophylaxis-review-clinical-effectiveness. Accessed 2022 Nov 7. [PubMed: 30160869] - 21.
- Zareifopoulos N, Lagadinou M, Karela A, Kyriakopoulou O, Velissaris D. Neuropsychiatric Effects of Antiviral Drugs. Cureus. 2020;12(8):e9536-e9536. [PMC free article: PMC7465925] [PubMed: 32905132]
Appendix 1. Selection of Included Studies
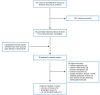
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Note that this appendix has not been copy-edited.
Table 2
Characteristics of Included Overview of Reviews.
Table 3
Characteristics of Included Systematic Review.
Table 4
Characteristics of Included Primary Clinical Studies.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Table 5
Strengths and Limitations of Overview of Reviews Using AMSTAR 27.
Table 6
Strengths and Limitations of Systematic Review Using AMSTAR 27.
Table 7
Strengths and Limitations of Clinical Studies Using the Downs and Black Checklist.
Appendix 4. Main Study Findings
Note that this appendix has not been copy-edited.
Table 8
Summary of Findings by Outcome — Secondary Transmission.
Table 9
Summary of Findings by Outcome — Attack Rate.
Table 10
Summary of Findings by Outcome — Oseltamivir Prophylaxis Failure.
Table 11
Summary of Findings by Outcome — Rate of Hospitalization.
Table 12
Summary of Findings by Outcome — Mortality.
Appendix 5. References of Potential Interest
Note that this appendix has not been copy-edited.
- The Use of Antivirals for Influenza Prophylaxis: A Review of the Clinical Effectiveness. Ottawa (ON): CADTH. 2017. https://www.cadth.ca/use-antivirals-influenza-prophylaxis-review-clinical-effectiveness Accessed 2022 Nov 8. [PubMed: 30160869]
- Heneghan CJ, Onakpoya I, Jones MA, et al. Neuraminidase inhibitors for influenza: a systematic review and meta-analysis of regulatory and mortality data. Health Technol Assess. 2016;20(42):1-242. [PMC free article: PMC4904189] [PubMed: 27246259]
- Cheng HY, Chen WC, Chou YJ, Huang ASE, Huang WT. Containing influenza outbreaks with antiviral use in long-term care facilities in Taiwan, 2008-2014. Influenza Other Respi Viruses. 2018;12(2):287-292. [PMC free article: PMC5820419] [PubMed: 29341490]
- Singh D, Jiang D, Van Caeseele P, Loeppky C. The effect of timing of oseltamivir chemoprophylaxis in controlling influenza A H3N2 outbreaks in long-term care facilities in Manitoba, Canada, 2014-2015: a retrospective cohort study. Infect Control Hosp Epidemiol. 2018;39(8):955-960. [PubMed: 29893659]
- Ye M, Jacobs A, Khan MN, et al. Evaluation of the use of oseltamivir prophylaxis in the control of influenza outbreaks in long-term care facilities in Alberta, Canada: a retrospective provincial database analysis. BMJ Open. 2016;6(7):e011686. [PMC free article: PMC4947728] [PubMed: 27381211]
- Jones M, Tett SE, Del Mar C. Psychiatric adverse events in oseltamivir prophylaxis trials: Novel comparative analysis using data obtained from clinical study reports. Pharmacoepidemiol Drug Saf. 2018;27(11):1217-1222. [PubMed: 30209862]
- Yip JLY, Kapadia S, Ahmed A, Millership S. Outbreaks of influenza-like illness in care homes in the East of England: impact of variations in neuraminidase inhibitor provision. Public Health. 2018;162:98-103. [PubMed: 29990618]
- Guidelines for the use of Oseltamivir in Long Term Care Facilities. Winnipeg (MB): Winnipeg Regional Health Authority. 2021. https:
//professionals .wrha.mb.ca/old/extranet /ipc/files/guideline-use-of-oseltamivir-in-ltc.pdf Accessed 2022 Nov 8. - Use of Oseltamivir (Tamiflu®) for the Management of Influenza Outbreaks in Special Care Homes. Regina (SK): eHealth Saskatchewan. 2017. https://www
.ehealthsask .ca/services/manuals /Documents/cdc-section-9-AntiviralUseforInfluenzaOutbreaksinSpecialCareHomes .pdf Accessed 2022 Nov 8. - Tzouvelekis A, Bouros D. CDC and PHE recommendations for the antiviral treatment and prophylaxis of influenza. Pneumon. 2016;29(4)():282-287.
- Lehnert R, Pletz M, Reuss A, Schaberg T. Antiviral Medications in Seasonal and Pandemic Influenza. Dtsch. 2016;113(47):799-807. [PMC free article: PMC5240024] [PubMed: 28043323]
- Smith LE, D'Antoni D, Jain V, Pearce JM, Weinman J, Rubin GJ. A systematic review of factors affecting intended and actual adherence with antiviral medication as treatment or prophylaxis in seasonal and pandemic flu. Influenza Other Respi Viruses. 2016;10(6):462-478. [PMC free article: PMC5059947] [PubMed: 27397480]
Previous CADTH Reports
Systematic Reviews
Non-Randomized Studies
Guidelines and Practice Recommendations
Review Articles
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up to date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to Requests@CADTH.ca
- Key Messages
- Context and Policy Issues
- Research Question
- Methods
- Summary of Evidence
- Limitations
- Conclusions and Implications for Decision- or Policy-Making
- References
- Selection of Included Studies
- Characteristics of Included Publications
- Critical Appraisal of Included Publications
- Main Study Findings
- References of Potential Interest
- Oseltamivir for the Prevention of Influenza in Residents of Long-Term Care Facil...Oseltamivir for the Prevention of Influenza in Residents of Long-Term Care Facilities
Your browsing activity is empty.
Activity recording is turned off.
See more...
