Copyright © 2023 - Canadian Agency for Drugs and Technologies in Health. Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International licence (CC BY-NC-ND).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
- The transcatheter edge-to-edge valve repair (TEER) device (TriClip) might be more effective in reducing tricuspid regurgitation severity and improving quality of life compared to medical therapy alone.
- The TEER device (TriClip) may have little impact on all-cause mortality, hospitalization due to heart failure, and 6-minute walk tests compared to medical therapy alone.
- The TEER device (TriClip) demonstrated a numerically higher rate of adverse events than a medical therapy control group in a clinical trial.
- Patients treated with the TEER device (TriClip) had fewer major adverse events (ranging from 2.5% to 7.1%) than the 10% performance goal in the included clinical trial; common major adverse events included major bleeding, single leaflet device attachment, and nonelective cardiovascular surgery. No device embolization or thrombosis were reported.
- We did not identify any studies that met our inclusion criteria to evaluate the effectiveness of the TEER device (TriClip) compared to open heart surgery.
Context and Policy Issues
What Is Tricuspid Regurgitation?
The tricuspid valve is positioned between the right atrium and the right ventricle, which is the largest cardiac valve with a normal orifice area between 7 cm2 and 9 cm2 and can be divided into the leaflets, the papillary muscles, the chordal attachments, and the annulus (with attached atrium and ventricle).1 The tricuspid valve usually has 3 different-sized leaflets, including septal, anterior-superior, and inferior,2 but some healthy people may have 2 or more than 3 leaflets.1 During systole, the leaflets, chordal attachments, and papillary muscles work together to facilitate tricuspid valve closure.
Tricuspid regurgitation (TR), also known as tricuspid valve regurgitation or tricuspid insufficiency, occurs when the tricuspid valve fails to close completely, resulting in blood flowing in the wrong direction (i.e., from the right ventricle to the right atrium). A TR diagnosis can be classified as either primary (or organic) TR or secondary (or functional) TR based on the underlying cause of disease.3 Primary (or organic) TR is rare and is a result of a primitive defect in the tricuspid valve caused by congenital or acquired conditions that affect the tricuspid valve or the subvalvular apparatus.1,3 Secondary (or functional) TR is more prevalent than primary TR and occurs because of other diseases, such as left-side heart diseases and pulmonary hypertension.1,3 Typically, there is no intrinsic damage to the tricuspid valve itself in secondary (or functional) TR.
How to Manage Tricuspid Valve Regurgitation?
Patients with TR can often present as asymptomatic. TR may be unnoticed during a physical examination, but can be diagnosed and evaluated through echocardiography.4,5 Proper management of TR requires health care specialists to assess and treat the underlying cause of the disease.6 Medical therapies, such as diuretics, and tricuspid valve surgery (including tricuspid valve repair or replacement) are options for treating TR. Valve replacement is considered to have a lower risk of recurrent TR than valve repair, but when considering operative mortality, technical ease, and speed of operation repair, valve repair is generally preferred, if possible.6,7 Transcatheter systems can be used for both valve repair and replacement. Studies have shown that patients at high risk of adverse events or complications due to open surgery may benefit from transcatheter tricuspid valve repair, which has demonstrated better clinical outcomes when compared to surgical tricuspid valve replacement or repair.8,9 Transcatheter tricuspid valve repair systems can either use edge-to-edge technology and an annuloplasty-based procedure. The edge-to-edge method appears to have a superior safety profile when compared to annuloplasty.10
What Is the Transcatheter Edge-to-Edge REPAIR?
Transcatheter edge-to-edge repair (TEER) is a minimally invasive procedure that treats valve leakage by using a catheter inserted through the femoral vein and delivering 1 or more clips with 2 arms to capture and lock valve leaflets without requiring open heart surgery.11 Abbott, a devices and health care company, developed the MitraClip to treat patients who are experiencing mitral regurgitation. Clinicians can use the MitraClip as a TEER device to treat TR, even though it is intended for mitral valve use. Following the launch of the MitraClip, Abbott introduced a modified version called the TriClip system, which is specifically meant for treating patients with TR.11 Another TEER system called PASCAL can also be used for treating TR. The PASCAL system builds on the success of the TriClip and other valve repair systems by combining a spacer to fill the regurgitant jet area, paddles to avoid stress concentration on native valve leaflets, and clasps for independent leaflet capture.12 To the best of our knowledge, Health Canada has only approved the TriClip as a TEER device for treating TR.13
Why Is It Important to Do This Review?
Clinicians have recently identified a gap in the treatment landscape for TR and considered it a “forgotten valve disease.”3 Patients with severe TR are at greater risk of mortality and adverse comorbidities and complications, including fatigue, weakness, and heart failure.3,14 In recent years, cardiology and research experts have emphasized that TR is an important prognostic factor for predicting clinical outcomes and an individual’s quality of life (QoL).14-16 Several systematic reviews have examined the clinical outcomes related to the use of transcatheter valve repair for treating TR.7-10 However, these reviews included both TEER devices (e.g., the MitraClip, TriClip, and PASCAL) and non-TEER devices (e.g., the FORMA repair system and cardioband) for treating TR. They were not designed to specifically focus on the TriClip system, nor provided a subgroup for it. Additionally, a randomized controlled trial (RCT) called the TRILUMINATE Pivotal trial (NCT03904147) has released its results on using the TriClip for TR management to the public.
Objectives
To support an evidence-based decision on the use of the TEER device (TriClip) in patients with TR, we summarize the latest evidence on the clinical effectiveness and safety of the TEER device (TriClip) for treating TR, including clinical trials and observational studies.
Research Question
What is the clinical effectiveness and safety of the TEER device (TriClip) for tricuspid valve regurgitation?
Methods
Literature Search Methods
An information specialist conducted a literature search on key resources, including MEDLINE, the Cochrane Database of Systematic Reviews, the International HTA Database, and the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search approach was customized to retrieve a limited set of results, balancing comprehensiveness with relevancy. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. Search concepts were developed based on the elements of the research questions and selection criteria. The main search concept was transcatheter tricuspid repair. CADTH-developed search filters were applied to limit retrieval to health technology assessments, systematic reviews, meta-analyses, or indirect treatment comparisons; as well as RCTs, controlled clinical trials, or any other type of clinical trial. The search was completed on June 30, 2023, and limited to English-language documents published since January 1, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
We excluded articles if they did not meet the selection criteria outlined in Table 1, they were duplicate publications, or were published before 2018. We also excluded studies that used unclear devices in the intervention, did not focus on the TriClip, or did not have a subgroup for using the TriClip for treating TR.
Critical Appraisal of Individual Studies
The included publications were critically appraised by 1 reviewer using the Downs and Black checklist17 for randomized and nonrandomized studies. Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Summary of Evidence
Quantity of Research Available
A total of 528 citations were identified in the literature search. Following screening of titles and abstracts, 489 citations were excluded and 39 potentially relevant reports from the electronic search were retrieved for full-text review. Six potentially relevant publications were retrieved from the grey literature search for full-text review. Of these potentially relevant articles, 39 publications were excluded for various reasons, and 6 publications met the inclusion criteria and were included in this report. These comprised 1 RCT and 5 reports of 4 single-arm observational studies. Appendix 1 presents the PRISMA18 flow chart for study selection.
Additional references of potential interest are provided in Appendix 5.
Summary of Study Characteristics
This report included 5 primary studies, with a total of 6 publications,19-24 including 1 RCT (TRILUMINATE Pivotal trial)19 and 4 single-arm observational studies with 5 publications.20-24 We did not identify any health technology assessments or systematic reviews that met the inclusion criteria. Furthermore, we did not find any studies that reported emergency department visits after TEER intervention. The characteristics of the included publications are provided in Appendix 2.
RCT (TRILUMINATE Pivotal trial)
We included 1 RCT that examined the clinical effectiveness for TEER device (TriClip) compared to guideline-directed medical therapy for heart failure alone.19 The RCT was a multicentre 2-arm trial (TRILUMINATE Pivotal trial, NCT03904147, funded by Abbott) conducted in the US, Canada, and across various European countries. The trial inclusion criteria were used to recruit a cohort of adults with severe and symptomatic TR with a New York Heart Association (NYHA) functional class II, III, or IV. Patients were also categorized as being at intermediate or greater surgical risk (determined by a group of local certified specialists), had been receiving stable guideline-directed medical therapy for heart failure for at least 30 days before enrolment, and had no other cardiovascular condition that required interventional or surgical correction.19
In total, 350 patients were enrolled in the trial, 55% of whom were female, with the average age of a patient being 78 years.19 Patients in the intervention group received TEER, which used the TriClip tricuspid valve repair system, while patients in the control group were treated by guideline-directed medical therapy alone.19 The primary outcome in the study was a hierarchical composite outcome measure, which was defined in order as all-cause death, tricuspid-valve surgery, hospitalization for heart failure, and improvement in QoL.19 Additionally, data were provided on single outcomes such as death or tricuspid-valve surgery, hospitalization due to heart failure, QoL measurement using the Kansas City Cardiomyopathy Questionnaire (KCCQ), TR severity, 6-minute walk test, and major adverse events (MAEs). The study followed participants for up to 1 year.19
Single-Arm Observational Studies
We included 5 reports20-24 summarizing 4 single-arm observational studies that examined the clinical outcomes of patients with TR who received TriClip tricuspid TEER. Two publications23,24 provide results for the TRILUMINATE single-arm study, which was conducted before the TRILUMINATE Pivotal trial. Abbott supported both the TRILUMINATE single-arm study and the TRILUMINATE Pivotal trial, which used the TriClip for treating TR. Out of the 4 studies, 3 were prospective20,21,23,24 and 1 was retrospective.22 The bRIGHT study20 (an observational real-world study evaluating patients with severe tricuspid regurgitation who were treated with the Abbott TriClip device) was conducted in 26 research centres across Europe, the TRILUMINATE single-arm study with 2 reports23,24 was conducted in 21 sites in Europe and the US, while 1 study21 was carried out at a single site in Italy, and the final study22 was conducted in 4 Spanish centres retrospectively.
All 4 studies involved patients with symptomatic TR receiving medical treatment (most patients had an NYHA function class of III or IV) and with a TR severity of at least moderate.20-24 The studies included a higher percentage of females than males, ranging from 56% to 85% females.20-24 The median age of patients ranged from 75 years to 81 years.20-24 The sample size ranged from 13 patients21 to 511 patients.20 All patients received TEER with the TriClip tricuspid valve repair system. These studies reported on different clinical outcomes, such as procedure outcomes, QoL, all-cause mortality, NYHA functional class, 6-minute walk test distance, hospitalizations due to heart failure, adverse events, TR severity, and other echocardiographic parameters.20-24 The follow-up period for these studies ranges from 1 month to 1 year after the intervention.20-24
Additional details regarding the characteristics of the included publications are provided in Appendix 2.
Summary of Critical Appraisal
RCT (TRILUMINATE Pivotal trial)
The study’s objectives and inclusion and exclusion criteria were well-defined and outlined. The study conducted a sample size calculation based on the primary outcome and successfully achieved its target sample size. The characteristics of the study participants were described in detail, including age, sex, NYHA functional class, comorbidities, and co-interventions. The study participants were representative of patients with isolated TR, according to the trial authors. Baseline characteristics were matched well between the intervention and control groups. The study intervention and outcome measures were clearly described. The study conducted sensitivity analyses to support the robustness of their findings. To reduce performance bias, personnel who were not aware of the group assignments conducted follow-up, and echocardiography assessments and standardized scripts were used.
The study was funded by Abbott, which is the manufacturer of the device. The sponsor had a role in choosing the research sites, overseeing the trial, gathering data, and examining the outcomes. This could potentially result in performance bias that favours the TEER device (TriClip). Although the study conducted sensitivity analyses for missing data, it did not provide clear information on the characteristics of patients who were lost to follow-up. The study was also an open-label study (for patients and clinicians), which may affect patients’ perceptions of the QoL measures and are at risk of measurement or reporting bias. The P values for certain outcome measures, such as changes in NYHA functional class and adverse events, were not reported. We are unsure if the trial has enough power to detect a statistical difference in individual outcomes such as all-cause mortality or hospitalization due to heart failure.
The study’s primary end point was a hierarchical composite outcome that, in order, included all-cause mortality, tricuspid-valve surgery, hospitalization due to heart failure, and at least a 15-point improvement in KCCQ score within a year. However, the use of this composite outcome makes it difficult to interpret the effectiveness of the TEER device (TriClip) on individual clinical outcomes. The outcomes were summarized via a win ratio for the primary composite outcome and the authors used the Finkelstein-Schoenfeld method. The study-reported win ratio was primarily driven by an improvement in KCCQ scores among the patients who hadn’t had surgery or were hospitalized due to heart failure, a property of the win ratio when the outcomes at the top of the hierarchy are less rare. The number of wins from all-cause mortality or tricuspid-valve surgery (in favour of intervention), or hospitalization due to heart failure (in favour of control) were similar between the intervention and control groups (no statistical tests were provided). The importance of all-cause mortality and tricuspid-valve surgery may differ for patients with TR.
Single-Arm Observational Studies
Three prospective single-arm observational studies20,21,23,24 clearly reported study objectives, inclusion and exclusion criteria, intervention details, participant characteristics, outcome measures, and the main findings, but 1 retrospective single-arm study22 lacked a clear description of its objectives, and inclusion and exclusion criteria. All 4 studies used paired statistical analysis methods for comparing changes before and after the intervention.20-24 Two studies (with sample sizes of 13 and 34)21,22 did not receive any specific funding, while 2 larger studies (with sample sizes of 511 and 85)20,23,24 were funded by Abbott. This could potentially lead to publication bias due to the influence of the funding source. All studies used a before-and-after design without a control group.20-24 Due to the nature of the design, it is difficult to attribute the before-and-after changes to the effect of the TEER device (TriClip). It is assumed that all these studies are open-label studies, which could result in bias in outcome measures that favours the TEER device (TriClip), particularly for QoL measures.
Additional details regarding the strengths and limitations of the included publications are provided in Appendix 3.
Summary of Findings
Five primary studies, including 1 RCT (TRILUMINATE Pivotal)19 and 4 single-arm observational studies with 5 publications (including 2 publications reporting the TRILUMINATE single-arm study),20-24 provided evidence on the clinical effectiveness and safety of the TEER device (TriClip) for patients with TR. The TRILUMINATE Pivotal RCT estimated a relative effect of adding the TEER device (TriClip) to medical therapy compared to medical therapy alone,19 while the single-arm studies identified insights to potential changes postintervention compared to baseline.20-24 The longest follow-up among these studies was 1 year.
Appendix 4 presents the main study findings.
Procedural Outcomes
All eligible studies reported some procedural outcomes and indicated that the TEER device (TriClip) had high success rates for both implant and procedure. The implant success was defined as the device was delivered and deployed accurately without any complications during the procedure. The procedure success was defined as TR improved by at least 1 grade and no device-related adverse events. We have the following information to highlight:
TRILUMINATE RCT Composite Outcome
The TRILUMINATE Pivotal RCT19 reported a hierarchical composite outcome as the primary end point, which included all-cause mortality or tricuspid-valve surgery, hospitalization due to heart failure, and at least a 15-point improvement in the KCCQ score within a year. Based on the reported win ratio results, it can be inferred that the TEER device (TriClip) outperformed the control group in a statistically significant manner.19
TR Severity
All eligible studies enrolled all or mostly all patients (90% or more) with severe or worse TR and reported on TR severity.19-24 Two studies19,23 indicate that the TEER device (TriClip) was effective in reducing TR severity up to the 1-year follow-up.
- TRILUMINATE Pivotal RCT: The TEER device (TriClip) group had a statistically significantly higher proportion of patients with moderate or lower TR than the control group.19
Quality of Life
One RCT19 and 3 single-arm studies20,21,23,24 reported on the continuous QoL measures. Three19,20,23 of these studies also reported QoL as a categorical outcome. These studies indicate that the TEER device (TriClip) was effective in improving QoL.
- TRILUMINATE Pivotal RCT:19 The patients in the TEER device (TriClip) group demonstrated statistically significant improvement in QoL, as measured by KCCQ scores, compared to those in the control group (medical therapy alone). Additionally, more patients in the TEER device (TriClip) group experienced at least a 15-point improvement in KCCQ scores than those in the control group.
- Single-arm studies: The mean QoL scores, including KCCQ, EQ-5D, Short Form (36) Health Survey (SF-36) physical component, SF-36 mental component, were statistically significant improved20,21,23,24 and most patients experienced at least a 10-point improvement in KCCQ scores after intervention with the TEER device (TriClip).20,23,24
NYHA Functional Class
All eligible studies enrolled most patients with NYHA III or IV and reported on NYHA functional class.19-24 Two studies19,23 indicate that the TEER device (TriClip) was effective in improving NYHA functional class (from NYHA III or IV to NYHA I or II) up to the 1-year follow-up.
- TRILUMINATE Pivotal RCT: The TEER device (TriClip) group had a numerically higher proportion of patients with NYHA I or II at 1-year follow-up than the control group, but the statistical test results were not available.19
6-Minute Walk Test
One RCT19 and 1 single-arm study reported on the 6-minute walk test (6MWT).23,24 While the single-arm study23,24 revealed a statistically significant improvement in 6MWT distance after the TEER procedure with the TriClip compared to baseline, the TRILUMINATE Pivotal RCT found no statistically significant difference in 6MWT distance between the TEER device (TriClip) and control groups.19 Therefore, it appears that the TEER device (TriClip) may have minimal impact on 6MWT distance.
Hospitalization Due to Heart Failure
All eligible studies provided information about hospitalization due to heart failure.19-24 Although there were discrepancies in the reported findings among these studies, the use of the TEER device (TriClip) may result in little change to the 1-year rate of hospitalization due to heart failure.
- TRILUMINATE Pivotal RCT: The group treated with the TEER device (TriClip) had numerically more patients hospitalized due to heart failure compared to the control group, but a formal statistical test was not available (likely no significant statistical difference).19
- Single-arm studies: Following the intervention with the TEER device (TriClip), the rate of hospitalization due to heart failure was numerically lower than the previous year,20,22,23 but this comparison is based on small sample sizes and importantly is potentially biased due to the patient selection methods that preferred patients with longer duration of heart failure and the assumption that heart failure hospitalization did not change over time.
All-Cause Mortality
All eligible studies included information about death from any cause in their reports.19-24 The use of the TEER device (TriClip) did not demonstrate an improvement in the incidence of all-cause mortality within 1 year of follow-up:
- The TRILUMINATE Pivotal RCT19 reported that the group treated with the TEER device (TriClip) had a lower proportion of patients (9.4%) who died compared to the control group (10.6%), but there were no statistical test results available. A test of significant statistical difference between the intervention and control would likely fail to support evidence of a difference in mortality.
- Two single-arm studies21,22 (n = 34 and n = 13) reported no patient deaths during their follow-up period, which lasted either 3 months22 or 6 months, respectively.21 Another study reported 5 out of 511 deaths (1%) within 30 days of being treated with the TEER device (TriClip), 20 while another study reported 6 out of 84 (7.1%) deaths after 1 year of follow-up.23
Adverse Events
All eligible studies included information about MAEs at 30 days and other adverse events in their reports.19-24
- TRILUMINATE Pivotal RCT:19 Patients treated with the TEER device (TriClip) had a significantly lower proportion of serious medical events compared to the expected performance goal of 10% in the included RCT. The study reported a performance goal of 90% for patients who were free from MAEs at 30 days. However, it is unclear how the performance goal was determined. The study also analyzed the occurrence of adverse events, both serious and nonserious, and found that the intervention group had a higher rate of events than the control group, while the TEER device (TriClip) group had a lower rate of TV surgery or reintervention compared to the control group.19 However, no statistical test results were available for these comparisons.
- The single-arm observational studies20-24 have reported different percentages of patients experiencing MAEs, ranging from 0% to 7.1%. Additionally, various adverse events were reported, including major bleeding (0% to 11.9%), single leaflet device attachment (3.8% to 13%), and nonelective cardiovascular surgery related to the device (0.2%).
Echocardiographic Outcomes
All eligible studies19-24 provided information on various echocardiographic outcomes, including measures of TR (e.g., effective regurgitant orifice area, vena contracta width, regurgitant volume) and right heart remodelling (e.g., RV end-diastolic dimension, tricuspid annular diameter, right atrial volume, RV fractional area change). The results of these studies were mixed but generally showed that the TEER device (TriClip) mainly affected changes in TR measures rather than right heart remodelling measures.19-24 The TRILUMINATE Pivotal RCT19 reported substantial decreases in vena contracta width, effective regurgitant orifice area, and regurgitant volume compared to the control group, but no statistical test results were available for these comparisons. Table 12 presents a summary of these important echocardiographic outcomes.
Other Outcomes
Two single-arm studies22,23 compared data on the daily dose of diuretics after treatment with the TEER device (TriClip) to baseline, and their results were mixed. One study indicated that more than 50% of patients had reduced their diuretic dosage after 3 months with a statistically significant daily dose reduction.22 However, the other study reported that most patients had maintained the same dosage of diuretic after 1 year of follow-up.23
Limitations
Three19,20,23,24 of 5 eligible studies, including the TRILUMINATE Pivotal trial, the bRIGHT study, and the TRILUMINATE single-arm study, were funded directly by the device manufacturer (Abbott). The funder was involved in a number of decisions in the study, such as designing, supervising the conduct, collecting data, and analyzing the results. This could potentially lead to performance, publication, or other bias in favour of the TEER device (TriClip). Four20-24 of 5 eligible studies were single-arm observational studies; single-arm trials are generally not considered as confirmatory for efficacy and are subject to several limitations that complicate their interpretation; for example, it is not possible to distinguish between the effect of TEER as an intervention, a placebo effect, or natural history of the disease in the absence of a frame of reference for comparison. The TRILUMINATE Pivotal trial19 calculated its sample size and met its target, but was based on assumptions about the primary outcome only — a hierarchical composite outcome. The study did not reveal any significant differences on mortality or hospitalization for heart failure when examined separately. This suggests that the statistical difference in the primary composite outcome was driven by the improvement in KCCQ scores and the trial may not have had sufficient power to distinguish a statistical difference in mortality or hospitalization for heart failure.
The body of evidence also has substantial inconsistency or unclear reporting in QoL measures. Out of the 5 eligible studies, 419-21,23 of them reported measures of QoL. Meanwhile, 219,20 out of the 4 studies only reported QoL using the KCCQ, 1 study21 used EQ-5D, and another study23,24 used KCCQ, the SF-36 physical component, and the SF-36 mental component. Two studies19,20 also reported the percentage of patients who experienced at least a 15-point improvement, while 1 study23 reported the percentage of patients who experienced at least a 10-point improvement. There were no reports of psychometric properties or minimal important difference changes on QoL measures.
In this report, we also found some evidence gaps in the body of evidence. The eligible studies were followed up for a maximum of 1 year; thus, the impact of the TEER device (TriClip) on clinical outcomes in the long term (over 1 year) remains uncertain. Participants in the TRILUMINATE Pivotal trial19 will be followed for 5 years. The release of the 5-year follow-up results will provide valuable insights into the effectiveness of the TEER device (TriClip) in improving clinical outcomes, particularly in reducing mortality or hospitalization due to heart failure. We did not find any systematic reviews or health technology assessments that specifically focus on the TriClip, which is the only Health Canada–approved TEER device for treating TR. We also did not find any studies that reported emergency department visits or that compared the TEER device (TriClip) with open heart surgery on clinical outcomes.
Additionally, we did not identify any studies that specifically focused on patients in Canada. While the TRILUMINATE Pivotal RCT did include participants from Canada, no subgroup was formed based on settings.19 Other eligible studies,20-24 such as the bRIGHT study, were conducted in Europe or the US; therefore, it is unclear whether the findings of these studies can be generalized to settings in Canada.
Conclusions and Implications for Decision-Making or Policy-Making
The report included 5 eligible studies, with a total of 6 publications19-24 addressing the research question. One of the publications, TRILUMINATE Pivotal RCT,19 was included, along with 5 other publications20-24 relating to 4 single-arm observational studies. Two publications23,24 provided results for the TRILUMINATE single-arm study. The body of evidence suggests that the TEER device (TriClip) had a high success rate for both implantation and the procedure. The TRILUMINATE Pivotal RCT19 indicated that the TEER device (TriClip) may be more effective than medical therapy alone in reducing TR severity and improving QoL (measured by KCCQ score) and NYHA functional class. However, it had little impact on all-cause mortality, and hospitalization due to heart failure for up to 1 year of follow-up. One single-arm study (the TRILUMINATE single-arm study) reported a statistically significant improvement in 6MWT distance after intervention with the TEER device (TriClip) compared to baseline at both 6-months24 or 1-year follow-up;23 however, the TRILUMINATE Pivotal RCT19 found no statistically significant difference in 6MWT distance between the TEER device (TriClip) and control groups.
The evidence indicated that patients treated with the TEER device (TriClip) had fewer MAEs than the expected 10% performance goal in the included clinical trial.19-24 However, the TEER device (TriClip) group had a higher rate of adverse events than the control group (medical therapy alone) numerically.19 However, no statistical test results were available for the comparison. MAEs included major bleeding, single leaflet device attachment, and nonelective cardiovascular surgery related to the device, but no device embolization or thrombosis were reported.
When making decisions based on the body of evidence, it is important to consider factors such as patient selection and training or the TEER device (TriClip). Before applying the TEER device (TriClip) intervention, all eligible studies19-24 conducted a thorough echocardiographic assessment; such assessment will help clinicians select appropriate patients for the procedure. Given that the TEER device (TriClip) intervention is an invasive procedure and that most studies19,20,23,24 on it were funded by Abbott, the device manufacturer, we can assume that the clinicians who participated in these studies received sufficient training for the intervention. To achieve comparable treatment outcomes, it is important to ensure that future clinicians who will use the TEER device (TriClip) will receive similar training.
Further primary studies that have a longer follow-up period of more than 1 year and compare the effectiveness of the TEER device (TriClip) to open heart surgery are necessary for evaluate the effectiveness of the TEER device (TriClip) on mortality and hospitalization due to heart failure. Future independent studies may be needed to confirm these findings. Additionally, comprehensive systematic reviews with appropriate pooled data and health technology assessments with cost-effectiveness analyses that specifically focus on the TriClip system are also required for decision-making.
Abbreviations
- 6MWT
6-minute walk test
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- MAE
major adverse event
- NYHA
New York Heart Association
- QoL
quality of life
- RCT
randomized controlled trial
- SF-36
Short Form (36) Health Survey
- TEER
transcatheter edge-to-edge valve repair
- TR
tricuspid regurgitation
Contributor: Chris Kamel
References
- 1.
- Dahou A, Levin D, Reisman M, Hahn RT. Anatomy and Physiology of the Tricuspid Valve. JACC Cardiovasc Imaging. 2019;12(3):458-468. [PubMed: 30846121]
- 2.
- Muraru D, Hahn RT, Soliman OI, Faletra FF, Basso C, Badano LP. 3-Dimensional Echocardiography in Imaging the Tricuspid Valve. JACC Cardiovasc Imaging. 2019;12(3):500-515. [PubMed: 30846124]
- 3.
- Condello F, Gitto M, Stefanini GG. Etiology, epidemiology, pathophysiology and management of tricuspid regurgitation: an overview. Rev Cardiovasc Med. 2021;22(4):1115-1142. [PubMed: 34957757]
- 4.
- Hahn RT, Thomas JD, Khalique OK, Cavalcante JL, Praz F, Zoghbi WA. Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovasc Imaging. 2019;12(3):469-490. [PubMed: 30846122]
- 5.
- Catherine M Otto. Etiology, clinical features, and evaluation of tricuspid regurgitation. In: Post TW, ed. UpToDate. Waltham (MA): UpToDate; 2021: http://www
.uptodate.com. Accessed 2023 Jun 30. - 6.
- Catherine M Otto. Management and prognosis of tricuspid regurgitation In: Post TW, ed. UpToDate. Waltham (MA): UpToDate; 2023: http://www
.uptodate.com. Accessed 2023 Jun 30. - 7.
- Sarris-Michopoulos P, Macias AE, Sarris-Michopoulos C, et al. Isolated tricuspid valve surgery-Repair versus replacement: A meta-analysis. J Card Surg. 2022;37(2):329-335. [PubMed: 34751979]
- 8.
- Wang X, Ma Y, Liu Z, et al. Comparison of outcomes between transcatheter tricuspid valve repair and surgical tricuspid valve replacement or repair in patients with tricuspid insufficiency. J Cardiothorac Surg. 2023;18(1):170. [PMC free article: PMC10148428] [PubMed: 37120579]
- 9.
- Bocchino PP, Angelini F, Vairo A, et al. Clinical Outcomes Following Isolated Transcatheter Tricuspid Valve Repair: A Meta-Analysis and Meta-Regression Study. JACC Cardiovasc Interv. 2021;14(20):2285-2295. [PubMed: 34674867]
- 10.
- Alperi A, Avanzas P, Almendarez M, et al. Early and mid-term outcomes of transcatheter tricuspid valve repair: systematic review and meta-analysis of observational studies. Rev Esp Cardiol (Engl). 2023;76(5):322-332. [PubMed: 35662675]
- 11.
- Overtchouk P, Piazza N, Granada J, Soliman O, Prendergast B, Modine T. Advances in transcatheter mitral and tricuspid therapies. BMC Cardiovasc Disord. 2020;20(1):1. [PMC free article: PMC6945613] [PubMed: 31910809]
- 12.
- Besler C, Seeburger J, Thiele H, Lurz P. Treatment options for severe functional tricuspid regurgitation: indications, techniques and current challenges. ESC European J Cardiol Practice. 2018;16.
- 13.
- Health Canada. Regulatory Decision Summary - TRICLIP SYSTEM. 2020; https://hpr-rps
.hres .ca/reg-content/regulatory-decision-summary-medical-device-detail .php?lang=en&linkID =RDS10839. Accessed 2023 Jul 10. - 14.
- Wang N, Fulcher J, Abeysuriya N, et al. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. Eur Heart J. 2019;40(5):476-484. [PubMed: 30351406]
- 15.
- Fujisawa T, Kimura T, Ikemura N, et al. Effect of Tricuspid Regurgitation on the Reported Quality of Life and Subsequent Outcomes in Patients With Atrial Fibrillation. J Am Heart Assoc. 2022;11(8):e022713. [PMC free article: PMC9238472] [PubMed: 35383465]
- 16.
- Leone PP, Chiarito M, Regazzoli D, et al. Prognostic value of tricuspid regurgitation. Rev Cardiovasc Med. 2022;23(2):76. [PubMed: 35229567]
- 17.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [PMC free article: PMC1756728] [PubMed: 9764259]
- 18.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [PubMed: 19631507]
- 19.
- Sorajja P, Whisenant B, Hamid N, et al. Transcatheter Repair for Patients with Tricuspid Regurgitation. N Engl J Med. 2023;388(20):1833-1842. [PubMed: 36876753]
- 20.
- Lurz P, Besler C, Schmitz T, et al. Short-term Outcomes of Tricuspid Edge-to-Edge Repair in Clinical Practice. J Am Coll Cardiol. 2023;13:13. [PubMed: 37207923]
- 21.
- Carpenito M, Cammalleri V, Vitez L, et al. Edge-to-Edge Repair for Tricuspid Valve Regurgitation. Preliminary Echo-Data and Clinical Implications from the Tricuspid Regurgitation IMAging (TRIMA) Study. J Clin Med. 2022;11(19):23. [PMC free article: PMC9571515] [PubMed: 36233476]
- 22.
- Freixa X, Arzamendi D, Del Trigo M, et al. The TriClip system for edge-to-edge transcatheter tricuspid valve repair. A Spanish multicenter study. Rev Esp Cardiol (Engl). 2022;75(10):797-804. [PubMed: 35288060]
- 23.
- Lurz P, Stephan von Bardeleben R, Weber M, et al. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J Am Coll Cardiol. 2021;77(3):229-239. [PubMed: 33478646]
- 24.
- Nickenig G, Weber M, Lurz P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394(10213):2002-2011. [PubMed: 31708188]
Appendix 1. Selection of Included Studies
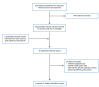
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Table 2
Characteristics of Included Primary Clinical Studies.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Table 3
Strengths and Limitations of Clinical Studies Using the Downs and Black Checklist.
Appendix 4. Main Study Findings
Note that this appendix has not been copy-edited.
Table 4
Summary of Findings by Outcome — Procedural Outcomes.
Table 5
Summary of Findings by Outcome — TR Severity.
Table 6
Summary of Findings by Outcome — Quality of Life.
Table 7
Summary of Findings by Outcome — New York Heart Association Functional Class.
Table 8
Summary of Findings by Outcome — 6-Minute Walk Test.
Table 9
Summary of Findings by Outcome — Hospitalization Due to Heart Failure.
Table 10
Summary of Findings by Outcome — All-Cause Mortality.
Table 11
Summary of Findings by Outcome — Adverse Events.
Table 12
Summary of Findings by Outcome — Echocardiographic Outcomes.
Appendix 5. References of Potential Interest
Note that this appendix has not been copy-edited.
- Wu Z, Zhu W, Kaisaier W, et al. Periprocedural, short-term, and long-term outcomes following transcatheter tricuspid valve repair: a systemic review and meta-analysis. Ther Adv Chronic Dis. 2023;14:20406223231158607. [PMC free article: PMC9989399] [PubMed: 36895329]
- Alperi A, Avanzas P, Almendarez M, et al. Early and mid-term outcomes of transcatheter tricuspid valve repair: systematic review and meta-analysis of observational studies. Rev Esp Cardiol (Engl). 2023;76(5):322-332. [PubMed: 35662675]
- Bocchino PP, Angelini F, Vairo A, et al. Clinical Outcomes Following Isolated Transcatheter Tricuspid Valve Repair: A Meta-Analysis and Meta-Regression Study. JACC Cardiovasc Interv. 2021;14(20):2285-2295. [PubMed: 34674867]
- Montalto C, Sticchi A, Crimi G, et al. Functional and Echocardiographic Improvement After Transcatheter Repair for Tricuspid Regurgitation: A Systematic Review and Pooled Analysis. JACC Cardiovasc Interv. 2020;13(23):2719-2729. [PubMed: 33189640]
- Nagaraja V, Kapadia SR, Miyasaka R, Harb SC, Krishnaswamy A. Contemporary review of percutaneous therapy for tricuspid valve regurgitation. Expert Rev Cardiovasc Ther. 2020;18(4):209-218. [PubMed: 32248715]
- Wang X, Ma Y, Liu Z, et al. Comparison of outcomes between transcatheter tricuspid valve repair and surgical tricuspid valve replacement or repair in patients with tricuspid insufficiency. J Cardiothorac Surg. 2023;18(1):170. [PMC free article: PMC10148428] [PubMed: 37120579]
- Wild MG, Low K, Rosch S, et al. Multicenter Experience With the Transcatheter Leaflet Repair System for Symptomatic Tricuspid Regurgitation. JACC Cardiovasc Interv. 2022;15(13):1352-1363. [PubMed: 35798479]
- Tanaka T, Kavsur R, Sugiura A, et al. Acute Kidney Injury Following Tricuspid Transcatheter Edge-to-Edge Repair. JACC Cardiovasc Interv. 2022;15(19):1936-1945. [PubMed: 36008268]
- Silini A, Guerin P, Jalal Z, et al. Percutaneous Edge-to-Edge Tricuspid Repair in Patients With Systemic Right Ventricle: A Multicenter French Cohort Study. JACC Cardiovasc Interv. 2023;16(2):240-242. [PubMed: 36697169]
- Kodali S, Hahn RT, Eleid MF, et al. Feasibility Study of the Transcatheter Valve Repair System for Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2021;77(4):345-356. [PubMed: 33509390]
- Miura M, Alessandrini H, Alkhodair A, et al. Impact of Massive or Torrential Tricuspid Regurgitation in Patients Undergoing Transcatheter Tricuspid Valve Intervention. JACC Cardiovasc Interv. 2020;13(17):1999-2009. [PubMed: 32912460]
- Kresoja KP, Lauten A, Orban M, et al. Transcatheter tricuspid valve repair in the setting of heart failure with preserved or reduced left ventricular ejection fraction. Eur J Heart Fail. 2020;22(10):1817-1825. [PubMed: 32741057]
- Cai S, Bowers N, Dhoot A, et al. Natural history of severe tricuspid regurgitation: Outcomes after transcatheter tricuspid valve intervention compared to medical therapy. Int J Cardiol. 2020;320:49-54. [PubMed: 32682962]
- Orban M, Rommel KP, Ho EC, et al. Transcatheter Edge-to-Edge Tricuspid Repair for Severe Tricuspid Regurgitation Reduces Hospitalizations for Heart Failure. JACC Heart Fail. 2020;8(4):265-276. [PubMed: 32241534]
- Taramasso M, Benfari G, van der Bijl P, et al. Transcatheter Versus Medical Treatment of Patients With Symptomatic Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2019;74(24):2998-3008. [PubMed: 31568868]
- Orban M, Orban MW, Braun D, et al. Clinical impact of elevated tricuspid valve inflow gradients after transcatheter edge-to-edge tricuspid valve repair. EuroIntervention. 2019;15(12):e1057-e1064. [PubMed: 31498114]
- Mehr M, Taramasso M, Besler C, et al. 1-Year Outcomes After Edge-to-Edge Valve Repair for Symptomatic Tricuspid Regurgitation: Results From the TriValve Registry. JACC Cardiovasc Interv. 2019;12(15):1451-1461. [PubMed: 31395215]
Systematic Reviews
Excluded for using broad intervention including other TEER systems and no subgroup or subsection for TriClip
Nonrandomized Studies
Excluded for using broad intervention including other TEER systems and no subgroup for TriClip
Using unclear or other TEER systems (e.g., MitraClip or PASCAL) for treating TR
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up to date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for noncommercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to Requests@CADTH.ca.
- Key Messages
- Context and Policy Issues
- Objectives
- Research Question
- Methods
- Summary of Evidence
- Limitations
- Conclusions and Implications for Decision-Making or Policy-Making
- Abbreviations
- References
- Selection of Included Studies
- Characteristics of Included Publications
- Critical Appraisal of Included Publications
- Main Study Findings
- References of Potential Interest
- Transcatheter Edge-To-Edge Valve Repair for Tricuspid RegurgitationTranscatheter Edge-To-Edge Valve Repair for Tricuspid Regurgitation
Your browsing activity is empty.
Activity recording is turned off.
See more...
