Copyright © 2023 - Canadian Agency for Drugs and Technologies in Health. Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International licence (CC BY-NC-ND).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
- Three guidelines recommend the use of dental sealants to prevent or manage dental caries in children and adolescents. Sealants may be used as a preventive measure upon the eruption of molars, or as treatment to arrest or reverse noncavitated carious lesions. Sealants may be used alone or in combination with other treatments that protect against tooth decay.
- Overall, the guidelines were rigorous, comprehensive, and clearly reported, but there were gaps in guidance about implementation, choice of sealant material, and selection of patients.
- There is a need for methodologically rigorous guidelines on dental sealants for the prevention of dental caries that are developed for use in Canadian settings.
Context and Policy Issues
Dental sealants, sometimes referred to as pit and fissure sealants, are coatings that are bonded to the chewing surfaces of the teeth.1 They may be resin-based, glass ionomer-based, or a combination of both materials (i.e., hybrid).2 Regardless of the material from which they are made, sealants are generally used to prevent caries, or tooth decay, from occurring in both primary and permanent teeth.1,2 When sealants are applied to surfaces of the teeth, they form a physical barrier that can help prevent buildup of food particles and colonization by cariogenic bacteria.1,3 Additionally, sealants can make teeth easier to clean, which is particularly important for pit and fissure surfaces as their anatomy makes cleaning difficult, even with attentive home care.2
While the evidence for the clinical effectiveness of dental sealants in primary teeth is limited,2 findings from a 2017 Cochrane systematic review4 suggested that sealants are effective in preventing dental caries in the permanent teeth of children and adolescents. Dental caries are a global issue that affect people of all ages, and so their prevention and effective treatment is crucial.5 The use of dental sealants for preventing dental caries also is important because untreated initial caries can result in a number of health issues including cavitation of the tooth, pain, inflammation, difficulty chewing, and loss of tooth.2 These outcomes can lead then lead to further health issues and negatively affect and individual’s quality of life.2,6 Furthermore, given the widespread nature of dental caries, failure to adequately intervene (e.g., with sealants) can result in a notable economic burden.2
Sealants are most often applied by dentists or dental hygienists.1 It is important to ensure that dental care providers are aware of the current best practices and procedures regarding dental sealants to ensure the greatest possible health outcomes for patients. The aim of the current report is to summarize and critically appraise the evidence-based guidelines regarding the use of dental sealants for the prevention of dental caries in children and adolescents.
Research Question
What are the evidence-based guidelines regarding the use of dental sealants for the prevention of dental caries in children and adolescents?
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including MEDLINE, the Cochrane Database of Systematic Reviews, the International HTA Database, the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were dental sealants and pediatrics. CADTH-developed search filters were applied to limit retrieval to guidelines. Where possible, retrieval was limited to the human population. The search was completed on January 16, 2023 and limited to English-language documents published since January 1, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in Table 1, they were duplicate publications, or were published before 2018. Guidelines were excluded if their methodology was unclear.
Critical Appraisal of Individual Studies
The included guidelines were critically appraised by 1 reviewer using the Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument7 as a guide. Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Summary of Evidence
Quantity of Research Available
A total of 77 citations were identified in the literature search. Following screening of titles and abstracts, 70 citations were excluded and 7 potentially relevant reports from the electronic search were retrieved for full-text review. Eight potentially relevant publications were retrieved from the grey literature search for full-text review. Of these potentially relevant articles, 12 publications were excluded for various reasons, and 3 evidence-based guidelines5,8,9 met the inclusion criteria and were included in this report. Appendix 1 presents the PRISMA10 flow chart of the study selection.
Additional references of potential interest are provided in Appendix 5.
Summary of Study Characteristics
Three evidence-based guidelines were included in this report.5,8,9
Additional details regarding the characteristics of included publications are provided in Appendix 2.
Study Design
The 3 guidelines were developed by the American Dental Association (ADA),5 the Scottish Dental Clinical Effectiveness Program (SDCEP),8 and a collaboration between the European Organization for Caries Research, the European Federation of Conservative Dentistry, and the German Association of Conservative Dentistry (referred to in this report as Splieth et al.).9
The ADA and SDCEP guidelines5,8 were published in 2018 and the guideline by Splieth and colleagues9 was published in 2020. The ADA and SDCEP guidelines5,8 each collected evidence to inform their recommendations using a systematic search of the literature. Randomized controlled trials were eligible for inclusion in the ADA guidelines,5 and systematic reviews and guidelines were eligible for inclusion in the SDCEP guidelines.8 The SDCEP guidelines also used systematic reviews from a previously developed guideline to inform their work.8 The recommendations made by the ADA and SDCEP were based on consensus.5,8 The ADA5 used a vote to resolve disagreement over recommendations. Splieth et al.9 obtained evidence from 3 hand-selected systematic reviews as well as a nonsystematic literature search. In addition to the hand-selected studies, the group included other systematic reviews and guidelines to help develop the guideline. Recommendations were based on a vote.
Recommendations included in the guideline developed by Splieth et al.9 were classified as strong (recommendations supported by consistent, clear evidence [e.g., many randomized controlled trials]), moderate (recommendations based on moderate evidence [e.g., high-quality clinical studies]), or weak (recommendations based on expert opinion only or those based on weak evidence [e.g., only low-quality studies or studies with contradicting results]). For the guideline developed by the ADA,5 evidence informing the recommendations was rated based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach as being of high, moderate, low, or very low certainty. The SDCEP guideline8 used the A MeaSurement Tool to Assess Systematic Reviews (AMSTAR) checklist to assess the quality of included systematic reviews and the AGREE II instrument to assess the quality of included guidelines. For both guidelines, the strength of recommendations were classified as either strong (most people would choose the recommended course of action) or conditional (the majority of people would choose the recommended course of action, but many would not).5,8
Country of Origin
The SDCEP guideline8 was developed for use in Scotland. The guideline by Splieth et al. and the ADA guideline do not report the country or region where they are meant to apply, but were developed by groups located across Europe9 and in the US,5 respectively.
Patient Population
The intended users of the guideline by Splieth et al.9 was dentists. The intended users of both the ADA and SDCEP guidelines included dental practitioners, other members of the primary care dental team, and those providing community dental education and coordination.5,7 The ADA guideline also noted policy-makers as potential users.5 The target population for the SDCEP guidelines was children from birth up to the age of 16 years.8 Similarly, the target population of the Splieth et al. guidelines was children (with no age range specified).9 The target population for the guidelines developed by the ADA included both children and adults.5 Only recommendations that apply to children 5 to 18 years of age were considered relevant to the current report.
Interventions
The guideline by Splieth et al.9 considered interventions for caries management in children, specifically regarding early childhood caries, caries in primary molars, and occlusal caries in the permanent teeth of children and adolescents. The SDCEP guideline8 considered a wide range of interventions for the prevention and management of dental caries including assessment, dental care management, delivery of preventive care, options for caries management, delivery of restorative care, referral, and management of suspected dental neglect. The ADA guideline5 considered nonrestorative interventions and treatments to arrest or reverse noncavitated and cavitated carious lesions.
Only the recommendations related to dental sealants were considered relevant for this report.
Outcomes
All guidelines considered the efficacy outcome of success of treatment in preventing dental caries when formulating recommendations.5,8,9 The ADA guidelines5 considered additional outcomes, including the arrest or reversal of noncavitated and cavitated carious lesions, nausea, fluorosis, vomiting, allergic reactions, staining, tooth sensitivity, soft-tissue trauma, progression of symptoms, pulpal health, lack of retention (for sealants), premature loss or extraction, and secondary caries.5 The guideline by Splieth et al. also examined pain as an outcome.9
Summary of Critical Appraisal
A narrative summary of strengths and limitations of the included studies is provided in this section. Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
All guidelines5,8,9 stated their overall objectives, described their relevant health questions, and indicated the populations to whom the guidelines were meant to apply. All guidelines5,8,9 also described target users and included individuals from relevant professional groups in the guideline development process. The guidelines developed by the ADA5 and SDCEP8 sought the views of the public and patients but Splieth et al.9 did not. To incorporate the views of the public, the SDCEP guideline8 was posted on the group’s website for comment for a period of 12 weeks. Additionally, 2 patient representatives were part of the guideline development group. Similarly, the recommendations from the ADA guideline5 were posted for review by the public on the ADA Centre for Evidence-Based Dentistry website. Both the ADA and SDCEP guideline development groups reviewed the comments received and incorporated feedback into their work as appropriate.5,8
The guidelines by the ADA and SDCEP used systematic methods to search for evidence, clearly described the criteria for selecting the evidence, and discussed the strengths and limitations of the body of evidence.5,8 This, in turn, increases the reproducibility and decreases the risk of bias in the selection of studies that were used to inform recommendations. For the ADA guideline,5 the full description of the systematic search and selection criteria are available in an associated systematic review discussed in the guideline. The ADA and SDCEP guidelines used validated tools to perform quality assessments like GRADE (used by the ADA) and AMSTAR and AGREE II (used by SDCEP).5,8 Splieth et al.9 hand-selected 3 systematic reviews and performed a nonsystematic literature search of the evidence to inform their recommendations. They included some description of their selection criteria and the strengths and limitations of the evidence; however, the description was minimal, thereby decreasing the reproducibility of the systematic review used to inform the guidelines. All 3 guidelines5,8,9 clearly described their methods for formulating recommendations. The ADA and SDCEP guidelines discussed health benefits and risks, described updating procedures, and were externally reviewed.5,8 However, these factors were not clearly reported or addressed in the Splieth et al. guideline.9 External reviewers of the SDCEP guideline8 included dentists in Scotland, organizations representing patient groups, and potential users of the guideline. External reviewers of the ADA guideline5 included numerous stakeholders, such as the Academy of Dental Materials, American Dental Hygienists’ Association, and the National Institute of Dental and Craniofacial Research, among other organizations. The link between the recommendations and supporting evidence was clearly presented in the guidelines by the ADA and SDCEP.5,8 The link between recommendations and the supporting evidence was only partially apparent in the Splieth et al. guideline.9 While the guideline provided some discussion of the evidence for each recommendation, the details of the associated studies (e.g., number of studies, number of participants, type of study) were not adequately described.
All guidelines5,8,9 were clear in their presentation. The recommendations were unambiguous, clearly identifiable, and various options for management of oral and dental health were discussed.
None of the guidelines described facilitators and barriers to their application. The SDCEP guideline8 noted that barriers to implementation of the recommendations were considered when developing the guideline, but specific examples were not given. The SDCEP guideline8 provided detailed explanations for how to put each recommendation into practice by providing associated images, instructions, and discussion of additional considerations for various populations (e.g., high risk versus standard risk) where applicable. The ADA5 guideline provided some flow charts to help end users visualize treatment pathways associated with the recommendations. However, it did not include additional details on the methods by which the recommendations can be put into practice, nor did it provide other resources such as educational tools or how to manuals. The guideline by Splieth et al.9 did not provide advice or tools for putting the recommendations into practice. Only the guideline by the ADA5 discussed potential costs and the consideration of alternatives for some of the recommendations, though discussion of human resources, such as staffing, were not mentioned. None of the guidelines discuss monitoring or auditing criteria.5,8,9
Editorial independence was ensured by all guidelines.5,8,9 Each publication provided a statement about the guideline development group’s conflict of interest and listed funding sources.
Overall, the methodology of the ADA and SDCEP guidelines5,8 were rigorous, comprehensive, and clearly reported. Despite the use of less rigorous methodology for identifying and selecting evidence, the guideline by Splieth et al.9 fulfilled many of the criteria in the AGREE II instrument.
Summary of Findings
Appendix 4 presents the detailed recommendations and supporting evidence that are relevant to the current report.
Recommendations Regarding the Use of Dental Sealants
The guideline by Splieth et al.9 recommends the use of preventive sealants in instances where there is a higher caries risk, activity, or prevalence (moderate recommendation). The guideline also recommends that preventive sealants be placed with low viscosity resin composites; however glass ionomer compound can be used when there are issues with moisture control (weak recommendation).9 Additionally, the guideline recommends that noncavitated occlusal caries lesions should be sealed if noninvasive care is not possible (weak recommendation).9 Finally, the guideline recommends the possibility of sealing remaining fissures of occlusal cavitated lesions following restoration with a filling, depending on the caries risk (weak recommendation).9 The authors did not report the quality of evidence associated with each recommendation.
The SDCEP guideline8 recommends that fissure sealants should be placed on permanent molars as early as possible for the prevention of dental caries (strong recommendation), based on moderate quality evidence.
The ADA guideline5 recommends the use of sealants alone or in combination with 5% sodium fluoride varnish over the use of other treatments to arrest or reverse noncavitated carious lesions on occlusal surfaces of primary or permanent teeth (strong recommendation), based on moderate certainty evidence. The guideline5 also recommends sealants alone as 1 possible treatment to arrest or reverse noncavitated carious lesions on approximal surfaces of primary and permanent teeth (conditional recommendation), based on low to very low certainty evidence.
Limitations
There were few guidelines meeting the inclusion criteria for this report were identified. The guidelines5,8,9 also lacked some specificity in their recommendations in terms of demographic information (e.g., the exact age that recommendations are applicable to) and only some of the recommendations mentioned the type of sealant material. Additionally, several of the recommendations were based on a low or very low quality of evidence, potentially limiting their applicability. Finally, none of the guidelines5,8,9 were developed in Canada, making the generalizability of the findings of this report to Canadian settings unclear.
Conclusions and Implications for Decision- or Policy-Making
Three evidence-based guidelines5,8,9 were included in this report. These guidelines recommend the use of dental sealants as preventive treatments for both dental caries and cavities in various clinical scenarios. The basis for these recommendations was evidence that ranged from very low quality to moderate quality. Overall, 2 of the included guidelines5,8 were highly rigorous, comprehensive, and clear in their methodology and reporting. One guideline9 used methods for identifying and selecting evidence that were less rigorous; however, it still met many of the criteria outlined in the AGREE II instrument.
Overall, the target population of each evidence-based guideline5,8,9 was broad and not particularly specific to subgroups within the pediatric population, making the recommendations generalizable to most users. However, because the guidelines were not specifically for use in Canada, the generalizability to Canadian settings was unclear.
A notable gap across the included guidelines was the discussion of facilitators and barriers to the guidelines’ application, as well as discussion of potential resource implications of applying recommendations. This, in turn, may make the implementation of the recommendations into practice more difficult. There were also gaps in recommendations about the use of specific materials for sealants (e.g., resin, glass ionomer compound), and guidance on patient selection in terms of appropriateness for sealants. This could leave room for uncertainty when applying these recommendations in clinical practice.
Higher quality clinical studies investigating the effectiveness of dental sealants for the prevention of dental caries could ultimately result in more robust recommendations. Future guidelines that are developed using rigorous methodology (including discussion on resource implications and the facilitators and barriers to applying the recommendations) and that are intended for use in Canadian settings may benefit clinicians and decision-makers. Finally, direction around the characteristics of patients appropriate for dental sealants as well as guidance on the optimal use of different sealant materials may be helpful for guideline users.
Abbreviations
- ADA
American Dental Association
- AGREE
Appraisal of Guidelines for Research and Evaluation
- AMSTAR
A MeaSurement Tool to Assess systematic Reviews
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- SDCEP
Scottish Dental Clinical Effectiveness Program
References
- 1.
- Nowak AJ WJ. Preventive dental care and counseling for infants and young children. In: Post TW, ed. Waltham (MA): UpToDate; 2022: www
.uptodate.com. Accessed 2023 Feb 7. - 2.
- Ramamurthy P, Rath A, Sidhu P, et al. Sealants for preventing dental caries in primary teeth. Cochrane Database Syst Rev. 2022(2). [PMC free article: PMC8832104] [PubMed: 35146744]
- 3.
- Heymann HO, Swift EJ, Ritter AV. Sturdevant's Art and Science of Operative Dentistry. 6th ed. St. Louis (MO): Elsevier-Mosby; 2013.
- 4.
- Ahovuo‐Saloranta A, Forss H, Walsh T, Nordblad A, Mäkelä M, Worthington HV. Pit and fissure sealants for preventing dental decay in permanent teeth. Cochrane Database Syst Rev. 2017(7). [PMC free article: PMC6483295] [PubMed: 28759120]
- 5.
- Slayton RL, Urquhart O, Araujo MWB, et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J Am Dent Assoc. 2018;149(10):837-849.e819. [PubMed: 30261951]
- 6.
- Seirawan H, Faust S, Mulligan R. The impact of oral health on the academic performance of disadvantaged children. Am J Public Health. 2012;102(9):1729-1734. [PMC free article: PMC3482021] [PubMed: 22813093]
- 7.
- Agree Next Steps C. The AGREE II Instrument. Hamilton (ON): AGREE Enterprise; 2017: https://www
.agreetrust .org/wp-content/uploads /2017/12/AGREE-II-Users-Manual-and-23-item-Instrument-2009-Update-2017.pdf. Accessed 2023 Feb 7. - 8.
- Prevention and management of dental caries in children: dental clinical guidance. Dundee (UK): Scottish Dental Clinical Effectiveness Programm; 2018: https://www
.sdcep.org .uk/media/2zbkrdkg/sdcep-prevention-and-management-of-dental-caries-in-children-2nd-edition.pdf. Accessed 2023 Jan 19. - 9.
- Splieth CH, Banerjee A, Bottenberg P, et al. How to Intervene in the Caries Process in Children: A Joint ORCA and EFCD Expert Delphi Consensus Statement. Caries Res. 2020;54(4):297-305. [PubMed: 32610317]
- 10.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [PubMed: 19631507]
Appendix 1. Selection of Included Studies
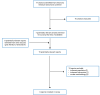
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Table 2
Characteristics of Included Guidelines.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Table 3
Strengths and Limitations of Guidelines Using AGREE II.
Appendix 4. Main Study Findings
Note that this appendix has not been copy-edited.
Table 4
Summary of Recommendations in Included Guidelines.
Appendix 5. References of Potential Interest
- Fluoride Varnish for the Prevention and Management of Dental Issues. Ottawa (ON): CADTH. 2022. https://www
.cadth.ca /fluoride-varnish-prevention-and-management-dental-issues Accessed 2023 Feb 7. - Silver Diamine Fluoride and Fluoride Varnish for the Prevention and Arresting of Dental Caries in Pediatric Populations: Clinical Effectiveness and Guidelines. Ottawa (ON): CADTH. 2020. https://www
.cadth.ca /silver-diamine-fluoride-and-fluoride-varnish-prevention-and-arresting-dental-caries-pediatric Accessed 2023 Feb 7. - American Academy of Pediatric Dentistry. Adolescent oral health care. The Reference Manual of Pediatric Dentistry. Chicago, Ill.: American Academy of Pediatric Dentistry; 2022:282-91.
- American Academy of Pediatric Dentistry. Caries-risk assessment and management for infants, children, and adolescents. In: The Reference Manual of Pediatric Dentistry. Chicago (IL): American Academy of Pediatric Dentistry; 2022:266-72.
- American Academy of Pediatric Dentistry. Periodicity of examination, preventive dental services, anticipatory guidance/ counseling, and oral treatment for infants, children, and adolescents. In: The Reference Manual of Pediatric Dentistry. Chicago (IL): American Academy of Pediatric Dentistry; 2022:253-65.
- Holve S, Braun P, Irvine JD, et al. Early childhood caries in Indigenous communities. Paediatr Child Health. 2021;26(4):255-256. [PMC free article: PMC8194778] [PubMed: 34136055]
Previous CADTH Reports
Guidelines and Recommendations
Best Practices Summary; Unclear Methodology
Alternative Population – Children Under 5 Years of Age
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up to date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to Requests@CADTH.ca
- Key Messages
- Context and Policy Issues
- Research Question
- Methods
- Summary of Evidence
- Limitations
- Conclusions and Implications for Decision- or Policy-Making
- Abbreviations
- References
- Selection of Included Studies
- Characteristics of Included Publications
- Critical Appraisal of Included Publications
- Main Study Findings
- References of Potential Interest
- Dental Sealants for the Prevention of Dental CariesDental Sealants for the Prevention of Dental Caries
Your browsing activity is empty.
Activity recording is turned off.
See more...
