Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Message
- Both included economic evaluation studies (1 from Iran and 1 from Australia) found that tenecteplase was the dominant treatment strategy (i.e., lower costs and higher benefit) compared with alteplase over a lifetime horizon.
Context and Policy Issues
There are approximately 878,000 people who currently live with stroke, and more than 89,000 strokes occur in Canada each year.1 Stroke is the third leading cause of death in Canada.1 The annual cost of acute care hospitalization for stroke in Canada is $146 million.1 The total cost of stroke to the Canadian economy is approximately $3.6 billion per year.1 There are 2 types of strokes: ischemic stroke, the most common form of stroke, which occurs when blood clots block blood vessels to the brain, and hemorrhagic stroke, which occurs when a blood vessel in the brain ruptures causing bleeding in or around the brain.1 Another subclass of stroke, called a transient ischemic attack (also referred to as mini-stroke), is caused by a small clot that briefly blocks a blood vessel in the brain.1 A transient ischemic attack is a warning sign that a major stroke may occur.1 Approximately 1.9 billion brain cells die every minute during a stroke.1 Therefore, early recognition of the signs of stroke and early stroke treatment and proper care as soon as possible will lead to a better chance of survival and better recovery.1
Thrombolysis (a process using medications to break down blood clots in blood vessels) is an approved treatment for acute ischemic stroke (AIS), which is a sudden loss of blood flow to part of the brain that results in the loss of neurologic function.2 Patients may be eligible for thrombolytic treatment if AIS is diagnosed within 4.5 hours of the onset of stroke symptoms.2 Alteplase (ALT), a thrombolytic drug, is approved in Canada and indicated for treatment of AIS within 3 hours after the onset of stroke symptoms and after exclusion of hemorrhagic stroke.3 ALT is a recombinant tissue plasminogen activator that cleaves plasminogen to form plasmin, an enzyme involved in the degradation of fibrin clots.4 ALT is given as an IV infusion over a period of 1 hour at a recommended dose of 0.9 mg/kg (maximum of 90 mg).3 Research has identified several limitations of ALT, including that it has a relatively short half life (initial half life of less than 5 minutes5), requires a long infusion,6 and is associated with low incidence of reperfusion after large-vessel occlusion.7
Tenecteplase (TNK), a genetically modified variant of ALT, has a longer half life that allows for bolus administration8 and faster reperfusion.9 TNK has been indicated for treatment of acute myocardial infarction,10 but has yet to be approved for treatment of AIS in Canada. An economic evaluation in Greece showed TNK was cost-effective for acute myocardial infarction compared with ALT over a lifetime horizon.11 A study of off-label use of TNK for treatment of AIS suggested that TNK was safe and was potentially associated with improved functional outcomes compared with ALT.12 Evidence from the EXTEND-IA TNK (Tenecteplase Versus Alteplase Before Thrombectomy for Ischemic Stroke) randomized trial conducted in Australia showed that thrombolytic treatment with TNK at a dose of 0.25 mg/kg increased reperfusion and improved functional outcomes compared with ALT.13 A recent randomized trial in Canada (AcT) showed that TNK at a dose of 0.25 mg/kg was noninferior to ALT for treatment of AIS.14 Both trials13,14 included patients with AIS within 4.5 hours after symptom onset who met the standard-of-care criteria for IV thrombolysis. There were no differences in terms of symptomatic intracerebral hemorrhage and death between the TNK and ALT groups in both trials.13,14 Thus, it is necessary to determine the comparative cost-effectiveness of these 2 thrombolytic agents to see if there is a need for change in the guidelines and clinical practice in replacing ALT with TNK for treatment of AIS.
The aim of this report is to summarize the evidence regarding the cost-effectiveness of TNK compared with ALT for AIS.
Research Question
What is the cost-effectiveness of tenecteplase compared with alteplase for acute ischemic stroke?
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the International HTA Database, the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were tenecteplase and acute ischemic stroke. CADTH-developed search filters were applied to limit retrieval to economic studies. The search was completed on December 21, 2022, and limited to English-language documents published since January 1, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in Table 1 or were published before 2018.
Critical Appraisal of Individual Studies
The included publications were critically appraised by 1 reviewer using the Drummond checklist15 for economic evaluations. Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Summary of Evidence
Quantity of Research Available
A total of 30 citations were identified in the literature search. Following screening of titles and abstracts, 22 citations were excluded and 8 potentially relevant reports from the electronic search were retrieved for full-text review. No potentially relevant publications were found from the grey literature search. Of the 8 potentially relevant articles, 6 publications were excluded for various reasons. Two publications met the inclusion criteria and were included in this report. Appendix 1 presents the PRISMA16 flow chart of the study selection.
Summary of Study Characteristics
Additional details regarding the characteristics of included publications are provided in Appendix 2.
Study Design
In their economic evaluation study, Hajian et al. (2022)17 conducted a cost-utility analysis using a decision tree model and a Markov model to evaluate the cost-effectiveness of TNK versus ALT in managing AIS. The study was carried out from the payer’s perspective in Iran, with a lifetime horizon. The lifetime horizon was assumed to be a maximum of 30 years, given the hypothetical patient age of 60 years at baseline. A decision tree model was constructed to model the disease’s natural progression after receiving TNK or ALT for 90 days, during which time patients may or may not have received thrombectomy. Patients could experience 1 of 3 outcomes: no event, recurrent stroke, or death. The probabilities for recurrent stroke were obtained from the published literature; the probabilities of death for patients with stroke in Iran were from the Iran life tables from WHO. The Markov model assigned 1 of 7 poststroke health states to each patient using a modified Rankin Scale (mRS) (scores 0 to 6). It was assumed that the Markov model was irreversible, such that patients with recurrent stroke could stay in the same state or progress to a worse state. The annual transition probabilities between different health states were obtained from the cost-effectiveness study by Gao et al. (2020)18 that is also included in our report. In the mRS, a score of 0 is no disability, 1 is no significant disability despite symptoms, 2 is mild disability (unable to carry out all previous activities, but able to take care of own affairs without assistance), 3 is moderate disability (requires some help, but able to walk without assistance), 4 is moderately severe disability (unable to walk or unable to attend bodily needs without assistance), 5 is disability requiring constant care for all needs, and 6 is dead. Each health state was assigned a utility value, which was used to calculate quality-adjusted life-years (QALYs), as described in the study by Gao et al.18 Costs of hospitalization procedures were obtained from 3 hospital databases. Other nonhospital costs, such as costs associated with laboratory tests, visits, home nursing, rehabilitation, and medications, were also included in the analyses. All costs were expressed in Iran’s currency, rial (IRR), and converted to US dollars (US$1 = IRR42,000 in June 2022). Costs and benefits were discounted at 5% annually.
In their economic evaluation study, Gao et al. (2020)18 performed a cost-utility analysis of TNK for large-vessel ischemic stroke via 2 approaches: within-trial economic analysis of the EXTEND-IA TNK trial13 and long-term modelling to extrapolate the short-term outcomes. A Markov model with 7 health states representing 7 mRS scores was used to evaluate the long-term cost-effectiveness of TNK versus ALT. The initial health status of patients in the model was their initial health state at day 90. From day 91 over the rest of their lifetime, patients in each health state could face 1 of 3 possibilities: no event, recurrent stroke, or death. Patients with recurrent stroke could only transition to a state that was equal or worse than their current state. The study was carried out from the Australian health care system perspective, with the time horizon consistent with the trial follow-up (i.e., 90 days) and a lifetime horizon for long-term modelling. The source of the clinical data was the EXTEND-IA TNK trial.13 The source of costs included the key study site for costs related to the acute hospitalization, published literature, and government websites. QALY gains were calculated from the utility (EQ-5D-3L) weight mapped from the mRS at day 90. Costs and benefits were discounted at 3% annually for a lifetime horizon. All costs were expressed in Australian dollars.
Country of Origin
The included economic evaluation studies were conducted by authors in Iran17 and Australia.18
Patient Population
Patient characteristics in the study by Hajian et al. (2022)17 were not reported. The study only mentioned that the target population was a hypothetical cohort of AIS patients aged 60 years at baseline.
Patients in the study by Gao et al. (2020)18 were those eligible for IV thrombolysis within 4.5 hours after the onset of ischemic stroke with large-vessel occlusion on CT angiography. The median time from stroke onset to hospital arrival was 60 minutes for the TNK group and 72 minutes for the ALT group.
Interventions and Comparators
Both the included economic evaluation studies17,18 assessed the cost-effectiveness of TNK versus ALT. In the study by Hajian et al. (2022),17 TNK was assumed to be administered at a dose of 0.40 mg/kg and ALT at 0.9 mg/kg. In the study by Gao et al. (2020),18 TNK was administered at 0.25 mg/kg (maximum 25 mg) and ALT at 0.9 mg/kg (maximum 90 mg).
Outcomes
The main outcome in both economic studies17,18 was the incremental cost-effectiveness ratio (ICER) expressed as incremental cost per additional QALY gain. In the study by Hajian et al. (2022),17 a willingness-to-pay (WTP) threshold of US$2,756.70 (equivalent to the Islamic Republic of Iran’s gross domestic product per capita) was used to assess the sustainability of the ICER. In the study by Gao et al. (2020),18 the WTP per QALY was set at AU$50,000.
Summary of Critical Appraisal
Additional details regarding the strengths and limitations of included economic evaluation studies17,18 are provided in Appendix 3.
Both included economic evaluation studies,17,18 clearly stated the objective, the economic importance of the research question, the rationale for choosing the alternative comparators (i.e., TNK versus ALT), and the type of economic evaluation (i.e., cost-utility analysis) that was conducted. The analyses in both studies17,18 were carried out with a clear perspective and time horizon. For data collection, the source of the clinical effectiveness data in the study by Hajian et al. (2022)17 comparing TNK and ALT was not reported, whereas the source of the clinical effectiveness data in the study by Gao et al. (2020)18 was clearly stated. The study by Gao et al. (2020),18 a post hoc within-trial economic analysis, used data collected during the EXTEND-IA TNK trial which was open-label but blinded to outcome assessors.13 A limitation of the EXTEND trial was that its results applied to patients with ischemic stroke and large-vessel occlusion who were eligible to undergo thrombectomy,13 which represents approximately 13% of all patients with ischemic stroke.19 Both economic evaluation studies17,18 clearly reported the source of cost data and resource utilization. The estimations of utilities and QALYs were described in both studies.17,18 Both studies17,18 used a Markov model for economic evaluation of the long-term consequences of AIS. However, it was unclear whether the results of a follow-up of 90 days after treatment were long enough to be extrapolated to a lifetime horizon in the model. For the analysis and interpretation of results, both studies17,18 clearly stated the time horizon of costs and benefits, statistical tests and confidence intervals, justification for the choice of variables for sensitivity analysis, and the ranges over which the variables were varied. In both studies, costs and benefits were discounted at 5%17 and 3%18 annually for a lifetime horizon. However, the reason for the choice of discount rate was not stated. Both studies reported incremental analyses and presented major outcomes in a disaggregated and aggregated form. The economic evaluation studies17,18 used both deterministic (1-way) and probabilistic sensitivity analyses (PSA) to test the robustness of the base-case results. The conclusion in both studies17,18 was based on the data reported. With respect to data collection, the study by Hajian et al. (2022)17 was of moderate methodological quality (due to the use of a hypothetical AIS population and unclear source of clinical effectiveness data), whereas the study by Gao et al. (2020)18 was of good methodological quality. Both studies17,18 were of good methodological quality with respect to analysis and interpretation of results.
Summary of Findings
Appendix 4 presents the main study findings and authors’ conclusions.
Cost-Effectiveness of Tenecteplase for Acute Ischemic Stroke
The cost-utility analysis over a lifetime horizon in the study by Hajian et al. (2022)17 showed that TNK was associated with lower cost compared to ALT, with an incremental cost (US$) of −4,444.81, whereas TNK had a higher benefit than ALT, with an incremental QALY of 0.25. The calculated ICER (US$ per QALY) was −17,450.29, showing that TNK was the dominant strategy for managing AIS. The cost-effectiveness acceptability curve in PSA showed that TNK was dominant compared with ALT regardless of the selected threshold values.
In the study by Gao et al. (2020),18 the within-trial economic analysis (i.e., 90-day follow-up) showed TNK had a lower cost with an incremental cost (AU$) of −5,412, and had higher benefit (i.e., incremental QALY of 0.10) compared with ALT, indicating that TNK was the dominant treatment strategy in the short-term. PSA, using a WTP threshold of AU$50,000 per QALY, showed TNK had a 97.4% probability of being cost-effective compared with ALT within 90 days, and TNK had a 91.0% probability of being dominant over ALT. In the long-term modelling, TNK was also associated with lower lifetime cost than ALT (AU$96,350 versus AU$106,311) and greater benefit (QALY: 7.77 versus 6.48). Both the base-case analysis and PSA showed that TNK had a 100% probability of being cost-effective (i.e., dominant strategy).
Limitations
Both included economic evaluation studies17,18 had some limitations, including the assumptions of some parameters used in the model. For example, it was unclear what key assumptions were made in the analyses. Due to lack of information for the Iranian setting in the study by Hajian et al. (2022),17 some parameters, such as utility values and QALYs, relied on international evidence, which might not be applicable to the Iranian setting. The sources of clinical data and population characteristics were not clearly presented in the Iranian study, which makes it difficult to appraise the internal and external validity of the analysis.17 The study by Hajian et al. (2022)17 was carried out from the payer’s perspective in Iran, which is not applicable to the Canadian context. The study by Gao et al. (2020)18 made multiple assumptions for the parameters (e.g., the probabilities of recurrent stroke, probability of background mortality, health-related quality of life, and cost of managing stroke were assumed to be identical between groups) in the analyses, which may not reflect the real situation. However, deterministic sensitivity analysis and PSA were used to test the robustness of the results, and the results were consistent with the base-case analysis. Because this study18 was a post hoc economic analysis using clinical data derived from the EXTEND-IA TNK trial,13 the health-related quality of life used in the analysis were not measured during the study. Therefore, a mapping algorithm from published literature was used to estimate the utility at day 90 for each patient.18 Although treatment with TNK was associated with nominally lower costs than ALT, the difference did not reach statistical significance.18 Given the lower drug cost and reduced frequency of endovascular thrombectomy procedures with the TNK treatment, a larger sample size would be needed to demonstrate a significant difference in total costs. No relevant Canadian economic evaluations were identified; however, the study by Gao et al. (2020)18 was carried out from the Australian health care system perspective, which could be generalized to the Canadian health care system. Both economic evaluation studies17,18 did not consider the indirect costs (i.e., societal perspective) in their models.
Conclusions and Implications for Decision- or Policy-Making
This report identified 2 economic evaluation studies17,18 that used a cost-utility approach to compare the cost-effectiveness of TNK versus ALT in patients with AIS. Both studies17,18 found TNK was the dominant treatment strategy (i.e., lower cost and higher benefit) compared with ALT over a lifetime horizon. The Australian study18 estimated a total cost savings for the Australian health care system of AU$28 million in the short-term and another AU$19 million in the long-term. Because the clinical effectiveness data from the AcT trial14 in Canada were consistent with data from the EXTEND-IA trial,13 the cost-effectiveness of TNK treatment in the Australian study18 is likely to generalize to the Canadian health care system, providing TNK is less expensive than ALT in the Canadian market. Therefore, an economic evaluation from the Canadian health care perspective or from the Canadian societal perspective, with a lifetime horizon, would be useful to assess the cost-effectiveness of TNK in patients with AIS in Canada.
Abbreviations
- AIS
acute ischemic stroke
- ALT
alteplase
- ICER
incremental cost-effectiveness ratio
- mRS
modified Rankin Scale
- PSA
probabilistic sensitivity analysis
- QALY
quality-adjusted life-year
- TNK
tenecteplase
- WTP
willingness-to-pay
References
- 1.
- Heart & Stroke. Stroke Month 2022 Key Messages and Backgrounder for media. 2022; https:
//heartstrokeprod .azureedge.net/-/media /pdf-files/what-we-do /news/stroke-month-2022-backgrounder-for-media .ashx?la =en&rev=a88365a3f5a94dae9ebc8e8f4d0fc310#:~:text =More %20than%2089%2C000%20strokes %20occur,third %20leading%20cause%20of%20death .&text =dramatically %20over%20the%20past %20several%20decades %20and%20stroke%20continues %20to%20rise .&text=research %20breakthroughs%2C %20better%20awareness %20of,and%20enhanced %20access%20to%20care. Accessed 2023 January 13. - 2.
- Walter K. What Is Acute Ischemic Stroke? JAMA. 2022;327(9):885. [PubMed: 35230392]
- 3.
- Activase® rt-PA (alteplase for injection) lyophilized powder for injection - 50 mg and 100 mg [product monograph]. Mississauga (ON): Hoffmann-La Roche Limited; 2021 Oct 7: https://www
.rochecanada .com/PMs/Activase/Activase_AIS_PM_E .pdf. Accessed 2023 January 13. - 4.
- Drugbank. Alteplase. 2023 Jan 31; https://go
.drugbank.com/drugs/DB00009. Accessed 2023 January 13. - 5.
- Reed M, Kerndt CC, Nicolas D. Alteplase. Treasure Island (FL): StatPearls Publishing; 2022: https://www
.ncbi.nlm .nih.gov/books/NBK499977 /#:~:text=Alteplase %20converts%20plasminogen %20to%20the,half %2Dlife%20of%2072%20minutes. Accessed 2023 January 27. - 6.
- Amirazodi E, Shamsaei G, Rafie S, Kashipazha D, Hesam S. Comparison of Efficacy and Complication of Alteplase Injection in Acute Ischemic Stroke. Archives of Medical Laboratory Sciences. 2021;7:1-6.
- 7.
- Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41(10):2254-2258. [PubMed: 20829513]
- 8.
- Tanswell P, Modi N, Combs D, Danays T. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. 2002;41(15):1229-1245. [PubMed: 12452736]
- 9.
- Warach SJ, Dula AN, Milling TJ, Jr. Tenecteplase Thrombolysis for Acute Ischemic Stroke. Stroke. 2020;51(11):3440-3451. [PMC free article: PMC7606819] [PubMed: 33045929]
- 10.
- TNKase® - tenecteplase for injection powder for solution - 50 mg/Vial [product monograph]. Mississauga (ON): Hoffmann-La Roche Limited; 2018 Jan 17: https://www
.rochecanada .com/PMs/TNKase/TNKase_PM_E.pdf. Accessed 2023 January 13. - 11.
- Maniadakis N, Kaitelidou D, Siskou O, et al. Economic evaluation of treatment strategies for patients suffering acute myocardial infarction in Greece. Hellenic J Cardiol. 2005;46(3):212-221. [PubMed: 15981557]
- 12.
- Katsanos AH, Psychogios K, Turc G, et al. Off-Label Use of Tenecteplase for the Treatment of Acute Ischemic Stroke: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5(3):e224506. [PMC free article: PMC8972028] [PubMed: 35357458]
- 13.
- Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N Engl J Med. 2018;378(17):1573-1582. [PubMed: 29694815]
- 14.
- Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022;400(10347):161-169. [PubMed: 35779553]
- 15.
- Higgins JPT, Green S, editors. Figure 15.5.a: Drummond checklist (Drummond 1996). Cochrane handbook for systematic reviews of interventions. London (GB): The Cochrane Collaboration; 2011: http://handbook-5-1
.cochrane .org/chapter_15 /figure_15_5_a_drummond _checklist_drummond_1996.htm. Accessed 2023 January 27. - 16.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [PubMed: 19631507]
- 17.
- Hajian K, Abdi Dezfouli R, Darvishi A, Radmanesh R, Heshmat R. Tenecteplase in managing acute ischemic stroke: a long-term cost-utility analysis in Iran. Expert Rev Pharmacoecon Outcomes Res. 2022:1-11. [PubMed: 36420792]
- 18.
- Gao L, Moodie M, Mitchell PJ, et al. Cost-Effectiveness of Tenecteplase Before Thrombectomy for Ischemic Stroke. Stroke. 2020;51(12):3681-3689. [PubMed: 33023423]
- 19.
- Chia NH, Leyden JM, Newbury J, Jannes J, Kleinig TJ. Determining the Number of Ischemic Strokes Potentially Eligible for Endovascular Thrombectomy: A Population-Based Study. Stroke. 2016;47(5):1377-1380. [PubMed: 26987869]
Appendix 1. Selection of Included Studies
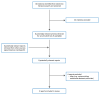
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Table 2
Characteristics of Included Economic Evaluations.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Table 3
Strengths and Limitations of Economic Evaluations Using the Drummond Checklist.
Appendix 4. Main Study Findings
Note that this appendix has not been copy-edited.
Table 4
Summary of Findings of Included Economic Evaluations.
Appendix 5. References of Potential Interest
- Dasari D, Deshmukh AA. Cost-Effectiveness Analysis of Tenecteplase Vs Alteplase for Treatment of Acute Ischemic Stroke. Value in Health. 2020;23(Supplement 1):S268.
- Thelengana A, Radhakrishnan DM, Prasad M, Kumar A, Prasad K. Tenecteplase versus alteplase in acute ischemic stroke: systematic review and meta-analysis. Acta Neurol Belg. 2019;119(3):359-367. [PubMed: 29728903]
- Oliveira M, Fidalgo M, Fontao L, et al. Tenecteplase for thrombolysis in stroke patients: Systematic review with meta-analysis. Am J Emerg Med. 2021;42:31-37. [PubMed: 33440328]
- Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N Engl J Med. 2018;378(17):1573-1582. [PubMed: 29694815]
- Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022;400(10347):161-169. [PubMed: 35779553]
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. [PubMed: 31662037]
- Warach SJ, Dula AN, Milling TJ, Jr. Tenecteplase Thrombolysis for Acute Ischemic Stroke. Stroke. 2020;51(11):3440-3451. [PMC free article: PMC7606819] [PubMed: 33045929]
Conference Abstract
Systematic Reviews
Randomized Controlled Trials
Guidelines and Recommendations
Review Articles
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to Requests@CADTH.ca
- Key Message
- Context and Policy Issues
- Research Question
- Methods
- Summary of Evidence
- Limitations
- Conclusions and Implications for Decision- or Policy-Making
- Abbreviations
- References
- Selection of Included Studies
- Characteristics of Included Publications
- Critical Appraisal of Included Publications
- Main Study Findings
- References of Potential Interest
- Tenecteplase for Acute Ischemic StrokeTenecteplase for Acute Ischemic Stroke
Your browsing activity is empty.
Activity recording is turned off.
See more...
