Copyright © 2023 - Canadian Agency for Drugs and Technologies in Health. Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International licence (CC BY-NC-ND).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
- Treatment with riluzole may provide clinical benefits for patients with amyotrophic lateral sclerosis, including longer survival time, reduced risk of disease progression, and minor reversible adverse events, compared to no treatment with riluzole or a placebo.
- We did not find any studies meeting our selection criteria on the clinical effectiveness of riluzole for patients with amyotrophic lateral sclerosis compared to alternative pharmacological therapies.
- Riluzole may be cost-effective at generic drug costs for patients with amyotrophic lateral sclerosis. Evidence supporting this finding is limited, and further research is required to inform decision-making.
- Riluzole is recommended for the treatment of amyotrophic lateral sclerosis, except for patients with progressive muscular atrophy, primary lateral sclerosis, or hereditary spastic paraplegia, and should be initiated promptly following disease diagnosis.
- A patient with lived experience of riluzole treatment for amyotrophic lateral sclerosis was involved in this report. They identified outcomes that are important to patients, including slowing the progression of amyotrophic lateral sclerosis and minimal side effects from the medication.
Context and Policy Issues
Roughly 1,000 individuals in Canada receive a diagnosis of amyotrophic lateral sclerosis (ALS) annually.1 At any time, around 2,500 to 3,000 people live with this disease in Canada. ALS is a fatal neurodegenerative disease characterized by the progressive degeneration of upper and lower motor neurons.2,3 Clinical features of ALS include limb paralysis, muscle atrophy, difficulty speaking or swallowing, shortness of breath, respiratory failure, and weakness.3 The most common presentation involves limb onset, with about 70% of patients experiencing extremity weakness and mobility impairment.2 Bulbar onset with oropharyngeal muscle involvement affecting swallowing and speech is observed in 25% of patients.2 In addition to motor impairment, up to 50% of patients may experience cognitive or behavioural impairments due to degeneration in the frontal and temporal lobes.2,4 Over time, strength progressively declines, and patients typically die from respiratory failure within 5 years of diagnosis.2 Despite an increase in research publications in recent years, treatment options for ALS remain limited, and patient care is primarily focused on symptom management and improving function and quality of life (QoL).2,5 Ventilation via a tracheostomy may be suitable for patients with ALS. It is estimated that less than 10% of those with ALS receive life-prolonging ventilation via a tracheostomy, specifically those who desire life-prolonging treatment.6 According to a study published in 2014 on the economic burden of ALS in Canada, the expenses associated with symptom management and care encompass both direct and indirect costs. Direct costs consist of expenditures on equipment, home renovations, medication, aids, and medical services. Indirect costs, on the other hand, include income loss, early retirement, and caregiver absence. The average annual direct and indirect costs per patient reported in this study were $32,337 (with 61% being out-of-pocket expenses) and $56,821, respectively.7 However, the average expenses for caring for a single person with ALS have also been reported to range from $150,000 to $250,000.1
Riluzole, a glutamatergic antagonist, is a disease-modifying treatment shown to extend survival time in ALS,8 demonstrating reduced mortality9 and improved survival time10 in past studies. A Health Canada Notice of Compliance (dated April 27, 2022) exists for riluzole for patients with ALS.11 According to the product monograph, based on evidence from older clinical trials (last updated July 9, 2012),12 riluzole is not a cure for ALS, but represents an initial step toward prolonging survival time.13 The recommended dosage for achieving a survival benefit, as observed in clinical trials, is 50 mg every 12 hours. There was limited experience with riluzole overdose in humans, but neurologic and psychiatric symptoms have been reported as side effects in isolated cases.13
The purpose of this report is to summarize and critically appraise recent available evidence (including observational studies) on the clinical effectiveness and cost-effectiveness of riluzole for patients living with ALS, as well as to summarize evidence-based guidelines regarding the use of riluzole for the treatment of ALS. A current understanding of the clinical effectiveness and cost-effectiveness of riluzole and guidelines for its use may contribute to informed decision-making, particularly in light of the emerging adjunctive and alternative drugs for ALS currently under development.
Research Questions
- What is the clinical effectiveness of riluzole for patients living with ALS compared to alternative pharmacological therapies?
- What is the clinical effectiveness of riluzole for patients living with ALS compared to no treatment?
- What is the cost-effectiveness of riluzole for patients living with ALS?
- What are the evidence-based guidelines regarding the use of riluzole for the treatment of patients with ALS?
Methods
Literature Search Methods
An information specialist conducted a literature search on key resources, including MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the International HTA Database, the websites of Canadian and major international health technology agencies, and a focused internet search. The search approach was customized to retrieve a limited set of results, balancing comprehensiveness with relevancy. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. Search concepts were developed based on the elements of the research questions and selection criteria. The main search concepts were riluzole (Rilutek) and amyotrophic lateral sclerosis. Comments, newspaper articles, editorials, letters, and conference abstracts were excluded. Retrieval was limited to the human population. The search was completed on May 5, 2023, and limited to English-language documents published since January 2011. This time frame was chosen as a Cochrane systematic review was published with a literature search completed in 2011.14
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in Table 1, were duplicate publications, or were published before January 1, 2011.
Critical Appraisal of Individual Studies
One reviewer critically appraised the included studies. The critical appraisal tools used were a Measurement Tool to Assess Systematic Reviews (AMSTAR-2)15 for systematic review (SRs) and meta-analyses (MAs), the Downs and Black checklist16 for randomized controlled trials (RCTs), the Drummond checklist17 for economic evaluations, and the Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument18 for guidelines. Summary scores were not calculated for the studies; rather, the strengths and limitations observed among the included studies were summarized and described narratively in Appendix 3.
Patient Engagement
CADTH has adopted the CADTH Framework for Patient Engagement in Health Technology Assessment,19 which includes standards for patient involvement in individual health technology assessments and is used to support and guide CADTH activities involving patients. For this report, CADTH engaged a patient contributor with lived experience with riluzole treatment for ALS.
Invitation to Participate and Consent
CADTH reached out to a patient advocacy group that supports people living with ALS. The preliminary engagement request included an overview of this project, the purpose of engagement, and the nature of engagement activities. An interested individual was identified, and the CADTH Patient Engagement Officer obtained the person’s informed consent to share with CADTH staff their lived experiences with ALS and the use of riluzole.
Engagement Activities
An individual contributor shared their personal experiences via video call during the drafting of the report. Patient perspectives gained through engagement processes were used to understand the relevance of reported outcomes in identified clinical effectiveness studies and to provide insights, background, and context to help inform the conclusion section.
Patient involvement was reported using the Guidance for Reporting Involvement of Patients and the Public (version 2) Short-Form reporting checklist20 (Table 22 in Appendix 6).
Summary of Evidence
Quantity of Research Available
A total of 519 citations were identified in the literature search. Following the screening of titles and abstracts, 496 citations were excluded and 23 potentially relevant articles from the electronic search were retrieved for full-text review. One potentially relevant publication was identified from the grey literature search for full-text reviews. Of these 24 potentially relevant articles, 12 were excluded because of the irrelevant intervention and/or comparator, and 122-5,14,21-27 met the selection criteria and were included in this report.
Appendix 1 presents the PRISMA28 flow chart of the study selection. Additional references of potential interest are provided in Appendix 5.
Summary of the Study Characteristics
Twelve peer-reviewed publications, including 1 SR with MA,14 8 observational studies,3-5,21-25 1 economic evaluation (i.e., cost-effectiveness study),27 and 2 guidelines2,26 were included in this report.
Additional details regarding the characteristics of the included publications are provided in Appendix 2 (Table 2, Table 3, Table 4, and Table 5).
Study Design
Of the 12 peer-reviewed publications that met the selection criteria for this report, 8 were observational studies3-5,21-25 (including 5 retrospective studies,4,21-25 2 prospective observational trials,3,5 and 1 analysis of a placebo RCT3) comparing patients who received riluzole with patients who did not receive riluzole or who received a placebo. The other 4 publications were 1 SR of RCTs with MA (search date from the databases’ inception to April 2011),14 1 cost-utility analysis,27 and 2 guidelines.2,26 For the study with cost-utility analysis, costs were quantified based on the health care sector and societal perspectives, and 5-year and 10-year time horizons were examined. A total of 4 reference case models were provided, representing the health care sector and societal perspectives at 5 years and 10 years. The cost-effectiveness threshold was set at $100,000 per quality-adjusted life-year (QALY) gained in the US context.27
Country of Origin
The authors of 1 SR with MA,14 1 economic evaluation,27 and 2 guidelines2,26 were based in the US,14,27 Sweden,26 and Canada.2 Eight observational studies3-5,21-25 were conducted in the US,21 UK,4 Iran,5 China,3 Austria,25 Taiwan,23 and Italy.24
Patient Population
In 1 SR with MA14 on patients who were diagnosed with ALS, of the 4 RCTs included, 1 study involved adults aged older than 75 years.29 The age range in other studies and the sex ratio in all included studies were not reported.
All 8 primary studies3-5,21-25 were carried out on male and female adults with a probable or definite ALS diagnosis. The studies had variable patient inclusion criteria related to age,21,22 riluzole use,3-5,21-25 Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) score at baseline,21,22 and time from onset to enrolment.21,22 The exclusion criteria were not reported in the studies.
In 1 economic evaluation study,27 a cost-utility analysis was conducted on the information of patients with ALS treated with riluzole in the authors’ institution.
Both guidelines2,26 included in this report were focused on patients diagnosed with ALS.
Interventions and Comparators
In 1 SR with MA,14 the intervention and comparator were treatment with oral riluzole and a placebo, respectively.
In all 8 primary studies,3-5,21-25 the intervention was riluzole and the comparators were no treatment with riluzole3,5,21-25 or a placebo.4
In the economic evaluation study,27 the intervention was riluzole and the comparator was usual supportive care for ALS, without riluzole use.
In the revised guideline of the European Federation of Neurological Associations (EFNS) on the clinical management of ALS,26 among a wide range of controlled therapeutic interventions, riluzole was the drug intervention relevant to this report. In the guideline on Canadian best practice recommendations for the management of ALS, among a variety of interventions included in the guideline (such as disease-modifying therapies; multidisciplinary care; respiratory management; nutritional management; medication alignment; interventions to manage venous thromboembolism, difficulty speaking, and other symptoms; exercise; cognition and behaviour management interventions; caregiver support, and palliative care), riluzole was 1 of the disease-modifying therapies relevant to this report.2
Outcomes
In 1 SR and MA,14 the primary outcome was a pooled hazard ratio (HR) based on percent mortality (or tracheostomy) for riluzole versus a placebo over all time points up to 12 months. Secondary outcomes were survival at 12 months and muscle strength and function evaluated by modified Norris Scales and adverse events (AE).
Outcome measures reported in the primary studies were survival rate,4,21,23-25 stage-specific risk of progression based on King and Fine’til 9 (FT9) staging methods,22 long-term (e.g., ranged from 36 months21 to 200 months3,25) riluzole effectiveness based on ALSFRS,3 and QoL using the ALS Assessment Questionnaire (ALSAQ-40).5
The ALSAQ-40 is a 40-item questionnaire, specifically designed to evaluate QoL in patients with ALS. The questionnaire contains 40 items divided into 5 sections, representing 5 distinct areas of health: mobility (10 items), activities of daily living (10 items), eating and drinking (3 items), communication (7 items), and emotional functioning (10 items). The questions refer to the patient’s condition during the past 2 weeks and the answers are given on a 5-point Likert scale, providing a summary score from 0 (best health status) to 100 (worst health status).30
In the economic evaluation study,27 the primary outcome was to examine the cost-effectiveness of riluzole in the treatment of ALS in regard to recent advances in disease staging and understanding of stage-specific drug effects. The authors used the FT9 staging method of ALS for estimating costs and utilities, and health states were described using the 3-Level EQ-5D.
Costs at 2018 prices obtained at the author’s institution from patients with ALS were disaggregated into recurring costs (RCs) and “one-off” transition and/or “tollgate” costs (TCs). Distributions of transition probabilities and utility weights were informed by direct estimation, whereas lognormal distributions were used for stage-specific RCs and TCs.
In the revised report of the EFNS task force on the clinical management of ALS,31 updating EFNS’s guidelines on the clinical management of ALS and identifying areas for further research were the main outcomes. In the guideline on Canadian best practice recommendations for the management of ALS,2 the primary outcome was to summarize best practice recommendations for the care and management of patients living with ALS in Canada, including all stages of the disease. This guideline aimed to establish a national standard to improve the quality of care for patients, families, and caregivers living with ALS.
Summary of the Critical Appraisal
Systematic Reviews
Of the 12 studies included in this report, 1 was an SR with MA to examine the efficacy of riluzole in prolonging survival, in delaying the use of tracheostomy to sustain survival, and to assess the efficacy of riluzole upon functional health.14 We used the a Measurement Tool to Assess Systematic Reviews (AMSTAR-2) checklist15 to evaluate the quality of the SR and determine whether the most important elements of the SR methodology were reported (Table 6).
The strengths of the SR were in defining the research question and inclusion and exclusion criteria, describing the study design of the selected primary studies, using a comprehensive literature search strategy, conducting study selection and data extraction in duplicate, using a satisfactory tool for assessing the risk of bias (RoB) in primary studies (based on the Cochrane Handbook for Systematic Reviews of Interventions) and reporting potential sources of conflict of interest. The methods for data synthesis to evaluate the time-to-tracheostomy primary outcome, which involved pooling weighted average HRs for each primary study, were appropriate. The authors applied life table methods to obtain summary survival curves for combined treated and combined control participants across different studies and doses, and the median survival for treated and control participants was estimated by interpolation from the survival curves. However, the SR did not include an explicit statement showing that the systematic search protocol was established before the conduct of the review and did not provide a list of excluded studies and justify the exclusions. The authors also did not describe the selected primary studies in detail, investigate publication bias (small study bias) in individual studies, and discuss its potential impact on the results of MA. Thus, the potential impact of studies with low sample sizes (which could result in more extreme treatment effects)32 on the findings was unclear. In addition, the SR did not report the sources of funding for the primary studies included in the review. Sources of funding for included primary studies are important for understanding the extent to which these may contribute to RoB in the findings, and the interpretation of their results (e.g., studies funded by private industry are at a higher RoB in favour of the intervention under study33).
Primary Studies
The Downs and Black Checklist used for critical appraisal of the included primary studies consists of 5 sections (Table 7): reporting, external validity, internal validity, confounding, and power. In the following paragraphs, the strengths and weaknesses of the included primary studies are described for each of the 5 sections.
Reporting: The items clearly described or reported in the 8 observational, nonrandomized studies3 to 5,21 to 25 (i.e., 5 retrospective studies,4,21-25 2 prospective observational trials,3,5 and 1 analysis of a placebo RCT3) included in this report were study objectives, characteristics of the patients (except 1 study5), interventions, estimated random variability in data for main outcomes (except 1 study25), actual P values for the main outcomes (except 1 study5), and characteristics of patients lost to follow-up. The main findings were not clearly described in 4 studies,4,5,21,22 and the distribution of potential confounders (e.g., sex, age, and disease characteristics) were not reported in 5 studies.3-5,23
External validity: Staff, places, and facilities described in the studies were representative of the procedures provided to patients, and funding sources were reported in most studies (except 3 studies).5,23,24 The primary studies included in this report were unclear if the patients who were asked to participate in the studies were representative of the entire population recruited (i.e., poor reporting of the source of the population).
Internal validity: The main outcome measures and statistical tests used for data analysis were clearly described in the studies (except for the unclear appropriateness of analyses in 2 studies).5,23 However, the studies did not report any information about adherence to medications. Thus, the number of patients who did not follow the prescribed regimen was unknown.
Confounding: In all studies it was unclear if losses of patients to follow-up were considered in statistical analyses, and it was unclear in most studies3-5,22,24 whether statistical analyses were adequately adjusted for potential confounding factors.
Power: Sample size and power calculation were not reported in the studies, and it was unclear if they were sufficiently powered to detect clinically significant effects, such as the survival advantage of riluzole in later stages of the disease25 and its impact on QoL.5
Additional details regarding the strengths and limitations of the included publications are summarized in Appendix 3.
Economic Evaluation
The 36-item Drummond checklist17 (Table 8) was used for critical appraisal of 1 study on the cost-effectiveness of riluzole for ALS included in this report.27 The areas questioned in the reporting checklist are related to the research question, description of the study and intervention, study design, measurements, valuation of costs and consequences, potential discounting, incremental analysis, presentation of results with uncertainty and sensitivity analyses, and discussion of results in the context of policy relevance and existing literature. The authors used appropriate self-reported measures of patients’ physical function (specifically, ALSFRS-Revised [ALSFR-R] scores) and QoL (EQ-5D scores) over time, along with the FT9 staging of ALS, to estimate costs and utilities using Markov models. The time horizons considered for these estimates were 5 years and 10 years, and the main model assumptions appeared to be appropriate;27 however, they did not provide adequate details of the patients from whom valuations were obtained, and productivity changes and their relevance to the study question. Productivity changes (losses) may contribute to a large proportion of costs of health conditions in an economic evaluation from a societal perspective,34 and using an appropriate methodology for measuring and valuing these productivity costs is essential.35 However, there is currently a lack of methodological consensus on how productivity losses should be measured and valued.34
Guidelines
The Appraisal of Guidelines for Research and Evaluation (AGREE) II Reporting Checklist18 (Table 9) was used for the critical appraisal of the 2 guidelines2,26 included in this report. The checklist is a tool to improve the completeness of reporting in clinical practice guidelines and is intended to provide guidance to guideline developers, guideline users, guideline funders, peer reviewers, and journal editors about the essential components of a high-quality practice guideline.36 The checklist entails 6 quality domains (scope and purpose, stakeholder involvement, rigour and development, clarity of presentation, applicability, and editorial independence) and 23 key items.36 The areas clearly described or reported in the 2 guidelines2,26 were overall objectives; main questions; target patients; the guideline development groups, including individuals from relevant health care professionals; the target users of the guidelines; the methods for formulating the recommendations; and the consideration of health benefits, side effects, and risks in formulating the recommendations. In both guidelines, key recommendations were easily identifiable, recommendations were specific and unambiguous, and different options for the management of patients with ALS were clearly presented. In addition, both guidelines included statements demonstrating that the views of the funding bodies have not influenced the content of the guideline, and competing interests of guideline development group members were mentioned. However, facilitators and barriers to the applications and monitoring and/or auditing criteria were the areas not covered in the guidelines. In the guideline developed by Shoesmith et al. (2020),2 it was unclear if a systematic method was used to search for evidence, and both guidelines were uncertain if the views of patients with ALS were sought. The guideline developed by Andersen et al. (2012)26 was unclear in several aspects, such as explaining how the quality of evidence supporting the recommendations was assessed and providing a link between the recommendations and their supporting evidence to ensure transparency and support the credibility of the recommendations. In addition, the guideline did not specify a procedure for updating its recommendations and did not indicate whether it underwent external expert review before publication. External review by subject matter experts is important to ensure the guideline’s rigour, accuracy, and relevance to the field.37
Summary of Findings
We identified 12 studies, including 1 SR with MA14 and 8 observational studies3-5,21-25 (5 retrospective studies,4,21-25 2 prospective observational trials,3,5 and 1 analysis of a placebo RCT3) on the clinical effectiveness of riluzole for patients with ALS, 1 study27 on the cost-effectiveness of riluzole for patients with ALS, and 2 evidence-based guidelines2,26 regarding the use of riluzole for the treatment of patients with ALS.
Appendix 4 presents the main outcomes of the included studies.
Clinical Effectiveness of Riluzole for Patients With ALS
Tracheostomy-Free Survival
In 1 SR with MA14 that reported pooled HRs, riluzole 100 mg per day was associated with a 9% gain in the probability of 1-year tracheostomy-free survival (49% in the placebo group and 58% in the riluzole group) and increased median tracheostomy-free survival from 11.8 months to 14.8 months compared to a placebo (Table 10). Improved survival time from disease onset to death or tracheostomy was not statistically significant in the Georgoulopoulou et al. (2013) study (Table 14).24
Survival
In the SR with MA,14 the pooled HR of mortality at 12 months with riluzole 100 mg was lower in patients who received riluzole than in those who received placebo. In the Thakore et al. (2022) study,21 median survival was significantly longer (i.e., 2 months) in patients treated with riluzole than in patients who did not receive riluzole, and 1-year treatment was associated with improved median survival by 1.9 months (Table 11). In the Georgoulopoulou et al. (2013) study,24 a significantly longer median survival time from onset to death (43 months versus 31 months) was reported in patients receiving riluzole than in patients not receiving riluzole (Table 14). A higher survival time23 (Table 13) and a survival advantage only for the initial treatment period or the early stage of the disease25 (Table 12) compared to no treatment with riluzole were reported in 2 other studies. In summary, the identified studies all reported a survival advantage for patients who received treatment with riluzole compared with those who did not.
Stage-Specific Risk of Progression
In the Thakore et al. (2020) study,22 adjustments were made for age and ALSFRS-R slope at the initial visit, and the probability of progression (represented by the transition from early to late stages and death based on the King and FT9 staging methods) with and without riluzole was evaluated. The reported HRs for both staging methods suggested the effectiveness of riluzole in reducing the risk of transitioning between different stages of ALS; however, the statistical significance of these findings was not reported (Table 15). Likewise, in the Fang et al. (2018) study,4 the time at stage 4 was significantly longer for patients receiving riluzole than for those receiving a placebo (Table 16).
Function and Muscle Strength
In 1 SR with MA,14 there was a small beneficial effect (i.e., a statistically significantly slower rate of decline) for riluzole versus a placebo on both bulbar and limb function (Table 10), but not on muscle strength (mean difference for rate of decline of muscle strength = −1.88; 95% confidence interval, −5.79 to 2.03).
Functional ability rated by ALSFRS and ALSFRS-R in 1 study3 showed significantly higher scores, which represented better functional ability, in patients treated with riluzole compared to patients not treated with riluzole (Table 17).
Cost-Effectiveness of Riluzole for Patients With ALS
In the economic evaluation study,27 from the health care sector perspective at the 5-year horizon, riluzole use contributed to 0.182 QALY gained at a cost difference of $12,348 ($5,403 riluzole cost; $8,870 RC and –$1,925 TC differences), demonstrating an incremental cost-effectiveness ratio of $67,658 per QALY. The study findings supported the cost-effectiveness of riluzole to treat ALS at the $100,000 per QALY threshold at generic drug costs (Table 19), and a conclusion of cost-effectiveness was maintained for analyses with a 10-year time horizon and from the societal perspective. Table 20 demonstrates the health state utility weights and costs (in dollars per month) by stage reported in this study.
Guidelines Regarding Riluzole Use for Patients With ALS
The authors of the 2 guidelines included in this report recommend that (Table 21):
- Patients with ALS should be offered treatment with riluzole 50 mg twice daily (based on evidence from RCTs), except for patients with progressive muscular atrophy, primary lateral sclerosis, or hereditary spastic paraplegia (based on expert consensus [EC]).26
- Treatment with riluzole is recommended soon after the diagnosis of ALS (based on EC), given its effectiveness in enhancing survival time by a median duration of 3 months.2
- Patients taking riluzole should be monitored for AEs (based on EC). In cases where patients experience fatigue while taking riluzole, the consideration of reducing or discontinuing the medication may be appropriate (based on EC and evidence from observational, nonrandomized studies).2
Limitations
The primary studies3-5,21-25 included in this report were at RoB from several aspects, such as not reporting the distribution of potential confounders (e.g., sex, age, and disease characteristics),3-5,23,27 compliance with the medications (riluzole), if losses of patients to follow-up were considered in statistical analyses,3-5,21-25 if the statistical analyses were adequately adjusted for potential confounding factors,3-5,22,24,27 and if statistical analyses were sufficiently powered to detect clinically significant effects. We did not find any studies that compare riluzole’s clinical effectiveness with alternative pharmacological drugs in patients with ALS.
In terms of the cost-effectiveness of riluzole for ALS, we did not identify any Canadian economic analyses that met the inclusion criteria for this review. In addition, the cost evaluation study included in this report27 was based on US health care perspectives, and the relevance of the information reported in this study to the Canadian health care system is unclear.
Conclusions and Implications for Decision- or Policy-Making
We reviewed a total of 9 studies (1 SR with MA14 and 8 primary studies3-5,21-25) on the clinical effectiveness of riluzole in patients with ALS compared to those not receiving riluzole3,21-25 or those who received a placebo.4,5,14 According to the SR with MA published in 2012,14 riluzole 100 mg was associated with 3 months longer median tracheostomy-free survival time than a placebo. Additionally, 6 studies published between 2013 and 2022 reported improved survival time21,23-25 and reduced risk of disease progression at different stages4,22 in patients treated with riluzole compared to those not receiving treatment22 or receiving a placebo.4
The SR and MA included in this report14 found that the AEs associated with riluzole were generally minor and reversible, such as nausea, asthenia, and an increase in serum alanine transferase. In addition, CADTH sought input from 1 patient with lived experience of early treatment with riluzole, who identified slowing disease progression and minimal side effects as priority outcomes for ALS treatment, which were aligned with the findings reported in this review.
Regarding functional ability, limited evidence suggests a small beneficial effect of riluzole compared to placebo on both bulbar and limb function,14 as well as improved functional ability compared to no treatment.3 In addition, we identified 1 study that reported no significant difference in QoL between treatment with riluzole and a placebo.5
We did not find any studies on the clinical effectiveness of riluzole for patients with ALS compared to alternative pharmacological therapies.
The findings of 1 economic evaluation study published in 202027 showed riluzole’s cost-effectiveness to treat ALS at the $100,000 per QALY threshold at generic drug cost, from a US health care system perspective.
According to 2 evidence-based guidelines,2,26 treatment with riluzole (50 mg twice daily) is recommended to treat ALS and should be initiated upon diagnosis,2,26 except for patients with progressive muscular atrophy, primary lateral sclerosis, or hereditary spastic paraplegia.26 While the guidelines did not comment on treatment delivery or implementation considerations in detail, the patient contributor to this report expressed a preference for the oral medication form due to its convenience (compared to infusions). The patient also highlighted potential barriers to access, such as the risk of losing medication access when requiring full-time continuous positive airway pressure (CPAP) therapy due to declining health.
The findings of the studies included in this report support the clinical effectiveness (i.e., longer survival time; reduced risk of disease progression; and minor, reversible AEs) of riluzole versus no treatment or a placebo for patients with ALS and suggest early treatment soon after disease diagnosis. Also, riluzole might be cost-effective at generic drug costs,27 though additional economic evaluation studies from a Canadian health care perspective are required to reach firm conclusions about the cost-effectiveness of riluzole for ALS and to facilitate informed decision-making. Furthermore, it is important to note that there are ongoing trials investigating the use of add-on drugs in conjunction with riluzole (Appendix 5) that have the potential to influence the role of riluzole in the treatment approach for ALS in the future. These additional interventions and the ongoing research highlight the dynamic nature of ALS treatment and the possibility of evolving treatment strategies beyond riluzole monotherapy.
Abbreviations
- AE
adverse event
- ALS
amyotrophic lateral sclerosis
- ALSFRS
Amyotrophic Lateral Sclerosis Functional Rating Scale
- ALSFRS-R
Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised
- EC
expert consensus
- EFNS
European Federation of Neurological Associations
- FT9
Fine’til 9
- HR
hazard ratio
- MA
meta-analysis
- QALY
quality-adjusted life-year
- QoL
quality of life
- RC
recurring cost
- RCT
randomized controlled trial
- RoB
risk of bias
- SR
systematic review
- TC
transition and/or “tollgate” cost
Contributors: Elizabeth Carson, Adeline Markarian, Farhana Shivji
Acknowledgement
Many thanks to patient contributor Scott Williams for his time and energy and for sharing his lived experiences and perspectives. His contribution was invaluable.
References
- 1.
- Amyotrophic Lateral Sclerosis Society of Canada. New Canadian investments in ALS research reflect growing knowledge about the disease and increasing likelihood of effective treatments being developed. Toronto (ON): ALS Society of Canada; 2016 Nov 23: https://als
.ca/blogs /new-canadian-investments-als-research-reflect-growing-knowledge-disease-increasing-likelihood-effective-treatments-developed/. Accessed 2023 May 25. - 2.
- Shoesmith C, Abrahao A, Benstead T, et al. Canadian best practice recommendations for the management of amyotrophic lateral sclerosis. CMAJ. 2020;16(192). [PMC free article: PMC7683000] [PubMed: 33199452]
- 3.
- Chen L, Liu X, Tang L, Zhang N, Fan D. Long-term use of riluzole could improve the prognosis of sporadic amyotrophic lateral sclerosis patients: a real-world cohort study in China. Front Aging Neurosci. 2016;8:246. [PMC free article: PMC5075535] [PubMed: 27822184]
- 4.
- Fang T, Al Khleifat A, Meurgey JH, et al. Stage at which riluzole treatment prolongs survival in patients with amyotrophic lateral sclerosis: a retrospective analysis of data from a dose-ranging study. Lancet Neurol. 2018;17(5):416-422. [PMC free article: PMC5899963] [PubMed: 29525492]
- 5.
- Shamshiri H, Fatehi F, Abolfazli R, et al. Trends of quality of life changes in amyotrophic lateral sclerosis patients. J Neurol Sci. 2016;368:35-40. [PubMed: 27538598]
- 6.
- Tysnes OB, Holmøy T, Indrekvam S, Fondenæs O. Ventilation of patients with amyotrophic lateral sclerosis. Tidsskriftet den Norske Legeforening. 2021 May 14: https:
//tidsskriftet .no/en/2021/05/kronikk /ventilation-patients-amyotrophic-lateral-sclerosis. Accessed 2023 Jun 5. - 7.
- Gladman M, Dharamshi C, Zinman L. Economic burden of amyotrophic lateral sclerosis: a Canadian study of out-of-pocket expenses. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(5-6):426-432. [PubMed: 25025935]
- 8.
- Zarate CA, Manji HK. Riluzole in psychiatry: a systematic review of the literature. Expert Opin Drug Metab Toxicol. 2008;4(9):1223-1234. [PMC free article: PMC2587133] [PubMed: 18721116]
- 9.
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591. [PubMed: 8302340]
- 10.
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425-1431. [PubMed: 8676624]
- 11.
- Health Canada. Notice of compliance [NOC] information: APO-Riluzole. 2022: https:
//health-products .canada.ca/noc-ac/nocInfo?no=28030. Accessed 2023 May 18. - 12.
- Health Canada. Product information. APO-Riluzole. 2023: https:
//health-products .canada.ca/dpd-bdpp /info?lang=eng&code=83784. Accessed 2023 May 18. - 13.
- PrAPO-Riluzole, riluzole tablets, USP, riluzole 50 mg [product monograph]. Toronto (ON): APOTEX INC; 2012: https://pdf
.hres.ca/dpd_pm/00017738.PDF. Accessed 2023 May 18. - 14.
- Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2012;2012(3):CD001447. [PMC free article: PMC7055506] [PubMed: 22419278]
- 15.
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [PMC free article: PMC5833365] [PubMed: 28935701]
- 16.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [PMC free article: PMC1756728] [PubMed: 9764259]
- 17.
- Higgins JPT, Green S, editors. Figure 15.5.a: Drummond checklist (Drummond 1996). Cochrane handbook for systematic reviews of interventions. London (GB): The Cochrane Collaboration; 2011: http://handbook-5-1
.cochrane .org/chapter_15 /figure_15_5_a_drummond _checklist_drummond_1996.htm. Accessed 2023 May 18. - 18.
- Agree Next Steps C. The AGREE II Instrument. [Hamilton, ON]: AGREE Enterprise; 2017: https://www
.agreetrust .org/wp-content/uploads /2017/12/AGREE-II-Users-Manual-and-23-item-Instrument-2009-Update-2017.pdf. Accessed 2023 May 18. - 19.
- CADTH framework for patient engagement in health technology assessment. Ottawa (ON): CADTH; 2019: https://www
.cadth.ca /cadth-framework-patient-engagement-health-technology-assessment. Accessed 2023 May 26. - 20.
- Staniszewska S, Brett J, Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. [PMC free article: PMC5539518] [PubMed: 28768629]
- 21.
- Thakore NJ, Lapin BR, Mitsumoto H, Pooled Resource Open-Access Als Clinical Trials Consortium. Early initiation of riluzole may improve absolute survival in amyotrophic lateral sclerosis. Muscle Nerve. 2022;66(6):702-708. [PMC free article: PMC9828202] [PubMed: 36117390]
- 22.
- Thakore NJ, Lapin BR, Pioro EP. Stage-specific riluzole effect in amyotrophic lateral sclerosis: a retrospective study. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(1-2):140-143. [PubMed: 31450993]
- 23.
- Lee CT, Chiu YW, Wang KC, et al. Riluzole and prognostic factors in amyotrophic lateral sclerosis long-term and short-term survival: a population-based study of 1149 cases in Taiwan. J Epidemiol. 2013;23(1):35-40. [PMC free article: PMC3700231] [PubMed: 23117224]
- 24.
- Georgoulopoulou E, Fini N, Vinceti M, et al. The impact of clinical factors, riluzole and therapeutic interventions on ALS survival: a population based study in Modena, Italy. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(5-6):338-345. [PubMed: 23373475]
- 25.
- Cetin H, Rath J, Fuzi J, et al. Epidemiology of amyotrophic lateral sclerosis and effect of riluzole on disease course. Neuroepidemiology. 2015;44(1):6-15. [PubMed: 25571962]
- 26.
- Andersen PM, Abrahams S, Borasio GD, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS): revised report of an EFNS task force. Eur J Neurol. 2012;19(3):360-375. [PubMed: 21914052]
- 27.
- Thakore NJ, Pioro EP, Udeh BL, Lapin BR, Katzan IL. A cost-effectiveness framework for amyotrophic lateral sclerosis, applied to riluzole. Value Health. 2020;23(12):1543-1551. [PubMed: 33248509]
- 28.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [PubMed: 19631507]
- 29.
- Bensimon G, Lacomblez L, Delumeau JC, Bejuit R, Truffinet P, Meininger V. A study of riluzole in the treatment of advanced stage or elderly patients with amyotrophic lateral sclerosis. J Neurol. 2002;249(5):609-615. [PubMed: 12021952]
- 30.
- Green C, Kiebert G, Murphy C, et al. Patients' health-related quality-of-life and health state values for motor neurone disease/amyotrophic lateral sclerosis. Qual Life Res. 2003;12(5):565-574. [PubMed: 13677501]
- 31.
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203-220. [PubMed: 15725977]
- 32.
- Turner RM, Bird SM, Higgins JP. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One. 2013;8(3):e59202. [PMC free article: PMC3609745] [PubMed: 23544056]
- 33.
- Fabbri A, Lai A, Grundy Q, Bero LA. The influence of industry sponsorship on the research agenda: a scoping review. Am J Public Health. 2018;108(11):e9-e16. [PMC free article: PMC6187765] [PubMed: 30252531]
- 34.
- Jiang S, Wang Y, Si L, et al. Incorporating productivity loss in health economic evaluations: a review of guidelines and practices worldwide for research agenda in China. BMJ Glob Health. 2022;7(8). [PMC free article: PMC9389102] [PubMed: 35977755]
- 35.
- Koopmanschap M, Burdorf A, Jacob K, Meerding WJ, Brouwer W, Severens H. Measuring productivity changes in economic evaluation: setting the research agenda. PharmacoEcon. 2005;23(1):47-54. [PubMed: 15693727]
- 36.
- AGREE reporting checklist. 2023: https://www
.agreetrust .org/resource-centre /agree-reporting-checklist/. Accessed 2023 May 16. - 37.
- Leung L. Validity, reliability, and generalizability in qualitative research. J Family Med Prim Care. 2015;4(3):324-327. [PMC free article: PMC4535087] [PubMed: 26288766]
- 38.
- Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169(1-2):13-21. [PubMed: 10540002]
- 39.
- Clinical review report: edaravone (radicava) (Mitsubishi Tanabe Pharma Corporation): indication: for the treatment of Amyotrophic Lateral Sclerosis (ALS). Appendix 4, validity of outcome measures.: Ottawa (ON): CADTH; 2019 https://www
.ncbi.nlm .nih.gov/books/NBK542356/. Accessed 2023 Jun 5. - 40.
- Balendra R, Al Khleifat A, Fang T, Al-Chalabi A. A standard operating procedure for King's ALS clinical staging. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(3-4):159-164. [PMC free article: PMC6558284] [PubMed: 30773950]
- 41.
- Bowden CL, Mintz J, Tohen M. Multi-state outcome analysis of treatments (MOAT): application of a new approach to evaluate outcomes in longitudinal studies of bipolar disorder. Mol Psychiatry. 2016;21(2):237-242. [PMC free article: PMC4573671] [PubMed: 25778474]
- 42.
- Maverick J. Recurring expenses vs. non-recurring expenses: what's the difference? Investopedia. 2021: https://www
.investopedia .com/ask/answers /072815/what-difference-between-recurring-and-nonrecurring-general-and-administrative-expenses.asp. Accessed 2023 Jun 5. - 43.
- Brainin M, Barnes M, Baron JC, et al. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces: revised recommendations 2004. Eur J Neurol. 2004;11(9):577-581. [PubMed: 15379736]
Appendix 1. Selection of Included Studies
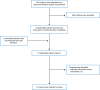
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Note that this appendix has not been copy-edited.
Table 2
Characteristics of the Included Systematic Review.
Table 3
Characteristics of Included Primary Clinical Studies.
Table 4
Characteristics of the Included Economic Evaluation.
Table 5
Characteristics of Included Guidelines.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Table 6
Strengths and Limitations of the Included Systematic Review Using a MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)15.
Table 7
Strengths and Limitations of Primary Clinical Studies Using the Downs and Black Checklist.
Table 8
Strengths and Limitations of the Included Economic Evaluation Study Using the Drummond Checklist.
Table 9
Strengths and Limitations of Guidelines Using the AGREE II Checklist.
Appendix 4. Main Study Findings
Note that this appendix has not been copy-edited.
Table 10
Summary of Evidence for Riluzole in Amyotrophic Lateral Sclerosis (ALS) in a Systematic Review and Meta-analysis by Miller et al. (2012).
Table 11
Summary of Findings by Outcome — Impact of Early Initiation of Riluzole on Median Survival in Thakore et al. (2022).
Table 12
Summary of Findings by Outcome — Survival Time (day) in Cetin et al. (2015).
Table 13
Summary of Findings by Outcome — Survival Time in Lee et al. (2015).
Table 14
Summary of Findings by Outcome — Survival Time in Georgoulopoulou et al. (2013).
Table 15
Summary of Findings by Outcome — Stage-Specific Riluzole Effect in Thakore et al. (2020).
Table 16
Summary of Findings by Outcome — Stage at Which Riluzole Treatment Prolongs Survival in Fang et al. (2018).
Table 17
Summary of Findings by Outcome — Long-Term Riluzole Effectiveness in Chen et al. (2016).
Table 18
Summary of Findings by Outcome — Quality of Life in Shamshiri et al. (2016).
Table 19
Summary of Findings of the Economic Evaluation Study — Cost-Effectiveness of Riluzole for Amyotrophic Lateral Sclerosis by Thakore et al. (2020)27.
Table 20
Summary of Findings of the Economic Evaluation Study — Health state utility weights and costs ($ per month) by stage in Thakore et al. (2020).
Table 21
Summary of Recommendations in the Included Guidelines.
Appendix 5. References of Potential Interest
The following publications were identified because they may provide additional information associated with this report.
- Chen PC, Hsieh YC, Huang CC, Hu CJ. Tamoxifen for amyotrophic lateral sclerosis: A randomized double-blind clinical trial. Medicine (Baltimore). 2020;99(22):e20423. [PubMed: 32481440]
- Ludolph AC, Schuster J, Dorst J, et al. Safety and efficacy of rasagiline as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomized, double-blind, parallel-group, placebo-controlled, phase 2 trial. Lancet Neurol. 2018;17(8):681-688. [PubMed: 29934198]
- Nagata E, Ogino M, Iwamoto K, et al. Bromocriptine mesylate attenuates amyotrophic lateral sclerosis: a phase 2a, randomized, double-blind, placebo-controlled research in Japanese patients. PLoS ONE. 2016;11(2):e0149509. [PMC free article: PMC4765990] [PubMed: 26910108]
- Elia AE, Lalli S, Monsurro MR, et al. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur J Neurol. 2016;23(1):45-52. [PMC free article: PMC5024041] [PubMed: 25664595]
- Shibuya K, Misawa S, Kimura H, et al. A single-blind randomized controlled clinical trial of mexiletine in amyotrophic lateral sclerosis: efficacy and safety of sodium channel blocker phase II trial. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(5-6):353-358. [PubMed: 25960085]
- Dupuis L, Dengler R, Heneka MT, et al. A randomized, double-blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PLoS ONE. 2012;7(6):e37885. [PMC free article: PMC3371007] [PubMed: 22715372]
- Verstraete E, Veldink JH, Huisman MH, et al. Lithium lacks effect on survival in amyotrophic lateral sclerosis: a phase IIb randomized sequential trial. J Neurol, Neurosurg Psychiatry. 2012;83(5):557-564. [PubMed: 22378918]
- Witzel S, Maier A, Steinbach R, et al. Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121-130. [PMC free article: PMC8749709] [PubMed: 35006266]
- Favero FM, Voos MC, Castro I, Caromano FA, Oliveira ASB. Epidemiological and clinical factors impact on the benefit of riluzole in the survival rates of patients with ALS. Arq Neuropsiquiatr. 2017;75(8):515-522. [PubMed: 28813081]
- Inoue-Shibui A, Kato M, Suzuki N, et al. Interstitial pneumonia and other adverse events in riluzole-administered amyotrophic lateral sclerosis patients: a retrospective observational study. BMC Neurol. 2019;19(1):72. [PMC free article: PMC6487018] [PubMed: 31029113]
- Stevic Z, Kostic-Dedic S, Peric S, et al. Prognostic factors and survival of ALS patients from Belgrade, Serbia. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(7-8):508-514. [PubMed: 27315438]
- AB Science. NCT03127267: Efficacy and Safety of Masitinib Versus Placebo in the Treatment of ALS Patients. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2023: https://clinicaltrials.gov/ct2/show/NCT03127267. Accessed 2023 Jun 09.
- Corestem, Inc. NCT04745299: Evaluation the efficacy and safety of mutiple lenzumestrocel (Neuronata-R® Inj.) treatment in patients with ALS (ALSummit). Bethesda (MD): U.S. National Library of Medicine; 2022: https://clinicaltrials.gov/ct2/show/NCT04745299?term=NCT04745299&draw=2&rank=1. Accessed 2023 Jun 09.
- Sanofi. NCT05237284: Phase 2 Study for SAR443820 in Participants With Amyotrophic Lateral Sclerosis (ALS) (HIMALAYA). Bethesda (MD): U.S. National Library of Medicine; 2023: https://clinicaltrials.gov/ct2/show/NCT05237284?term=NCT05237284&draw=2&rank=1. Accessed 2023 Jun 09.
- InFlectis BioScience. NCT05508074: Treatment Combining Riluzole and IFB-088 in Bulbar Amyotrophic Lateral Sclerosis (TRIALS Protocol). Bethesda (MD): U.S. National Library of Medicine; 2022: https://clinicaltrials.gov/ct2/show/NCT05508074?term=NCT05508074&draw=2&rank=1. Accessed 2023 Jun 09.
- Johnson SA, Fang T, De Marchi F, et al. Pharmacotherapy for amyotrophic lateral sclerosis: a review of approved and upcoming agents. Drugs. 2022;82(13):1367-1388. [PubMed: 36121612]
- Neupane P, Thada PK, Singh P, et al. Investigating edaravone use for management of Amyotrophic Lateral Sclerosis (ALS): a narrative review. Cureus. 2023;15(1):e33746. [PMC free article: PMC9922523] [PubMed: 36788871]
- Song Y, Jia Q, Guan X, et al. Herbal medicine for amyotrophic lateral sclerosis: a systematic review and meta-analysis. Front Pharmacol. 2022;13:946548. [PMC free article: PMC9473725] [PubMed: 36120351]
- Andrews JA, Jackson CE, Heiman-Patterson TD, Bettica P, Brooks BR, Pioro EP. Real-world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration. 2020;21(7-8):509-518. [PubMed: 32573277]
Primary Studies
Alternative Intervention and/or Comparator
No Comparator
Current Trials on Add-On Drugs to Riluzole
Reviews
Appendix 6. Guidance for Reporting Involvement of Patients and the Public (Version 2) Short-Form Reporting Checklist
Note that this appendix has not been copy-edited.
Table 22
Patient Involvement in Riluzole for the Treatment of ALS.
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up to date as of the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, collecting, using, and disclosing by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to Requests@CADTH.ca.
- Key Messages
- Context and Policy Issues
- Research Questions
- Methods
- Summary of Evidence
- Limitations
- Conclusions and Implications for Decision- or Policy-Making
- Abbreviations
- Acknowledgement
- References
- Selection of Included Studies
- Characteristics of Included Publications
- Critical Appraisal of Included Publications
- Main Study Findings
- References of Potential Interest
- Guidance for Reporting Involvement of Patients and the Public (Version 2) Short-Form Reporting Checklist
- Riluzole for Amyotrophic Lateral Sclerosis TreatmentRiluzole for Amyotrophic Lateral Sclerosis Treatment
Your browsing activity is empty.
Activity recording is turned off.
See more...
